27
A Vote “For” Proposal 2.
Proposal 3
Approval of the Increase in Number of Authorized Shares of Common Stock
GeneralExecutive Officers
The Board has approved an amendment (the “Authorized Shares Amendment”) to the Company’s Amended and Restated Certificate of Incorporation (the “Certificate of Incorporation”) to increase the number of authorized shares of common stock from 200,000,000 to 400,000,000. The Authorized Shares Amendment will not change the number of authorized shares of preferred stock, which currently consists of 10,000,000 shares of preferred stock.
The additional shares of common stock authorized for issuance by the Authorized Shares Amendment would be a part of the existing class of common stock and, if and when issued, would have the same rights and privileges as the common stock presently issued and outstanding. The full text of the proposed Authorized Shares Amendment is attached to this Proxy Statement as Appendix A. However, the text of the Authorized Shares Amendment is subject to revision to include such changes as may be required by the Secretary of State of the State of Delaware and as deemed necessary and advisable to effect the Authorized Shares Amendment.
Provided the stockholders approve the Authorized Shares Amendment, the increased number of shares would be authorized for issuance, but would remain unissued until such time as the Board approves a specific issuance of such shares. Other than future issuances under the Company’s equity compensation plans and issuances of common stock that may be issued and sold under our Common Stock Sales Agreement with Cowen and Company, LLC ($167.3 million of common stock remains available for sale under such agreement as of December 31, 2021), the Company currently has no plans or arrangements to issue the additional authorized shares of common stock that will result in the event that the Company’s stockholders approve, and the Company implements, the Authorized Shares Amendment.
Adoption of the Authorized Shares Amendment would not affect the rights of the holders of currently outstanding common stock, except for effects incidental to increasing the number of shares of our common stock outstanding, such as dilution of the earnings per share and voting rights of current holders of common stock, to the extent that any additional shares of common stock are ultimately issued out of the increase in authorized shares proposed in the Authorized Shares Amendment.
If the proposed Authorized Shares Amendment is approved by the requisite vote of the stockholders, it will become effective upon the filing of a Certificate of Amendment with the Secretary of State of the State of Delaware. The Board reserves its right to elect not to proceed with and abandon the Authorized Shares Amendment if it determines, in its sole discretion at any time, that this proposal is no longer in the best interests of our stockholders.
If we fail to obtain stockholder approval of this proposal at the Annual Meeting, we may continue to seek to obtain stockholder approval at each subsequent annual meeting of stockholders and/or special meetings of stockholders until such approval has been obtained and we will incur the costs associated therewith.
Background
In addition to the 143,569,902 shares of common stock outstanding on March 31, 2022, we have also reserved, as of March 31, 2022, 22,567,690 shares for issuance upon the exercise or vesting of outstanding stock awards and 19,889,713 shares for issuance pursuant to the Company’s equity incentive and employee stock purchase plans, meaning that we presently have 13,972,695 authorized shares available for issuance, which is insufficient to meet our needs in connection with future financings or strategic transactions and properly incentivizing our key personnel.
Purposes and Effects of the Authorized Shares Amendment
The Board is recommending the proposed increase in the authorized number of shares of common stock to provide the Company with appropriate flexibility to issue additional shares in the future on a timely basis if such need arises in connection with potential financings, business combinations or other corporate purposes. Approval of the Authorized Shares Amendment could enable the Company to take advantage of market conditions, the availability of more favorable financing, and opportunities for business combinations and other strategic transactions, without the potential delay and expense associated with convening a special stockholders’ meeting.
Our success also depends in part on our continued ability to attract, retain and motivate highly qualified management and key personnel. If this proposal is not approved by our stockholders, the lack of unissued and unreserved authorized shares of common stock to provide future equity incentive opportunities could adversely impact our ability to achieve these goals. In short, if our
stockholders do not approve this proposal, we may not be able to access the capital markets, complete corporate collaborations or partnerships, attract, retain and motivate employees, and pursue other business opportunities integral to our growth and success.
The proposed increase in the number of authorized shares of common stock will not, by itself, have an immediate dilutive effect on our current stockholders. However, if this proposal is approved, unless otherwise required by applicable law or stock exchange rules, the Board will be able to issue the additional shares of common stock from time to time in its discretion without further action or authorization by the stockholders. The newly authorized shares of common stock would be issuable for any proper corporate purpose, including capital raising transactions of equity or convertible debt securities, the establishment of collaborations or other strategic agreements, stock splits, stock dividends, issuance under current or future equity incentive plans, future acquisitions, investment opportunities, or for other corporate purposes. The future issuance of additional shares of common stock or securities convertible into our common stock may occur at times or under circumstances that could result in a dilutive effect on the earnings per share, book value per share, voting power and percentage interest of the present holders of our common stock, some of whom have preemptive rights to subscribe for additional shares that we may issue.
Potential Anti-Takeover Effect
An increase in the number of authorized but unissued shares of common stock relative to the number of outstanding shares of common stock may also, under certain circumstances, be construed as having an anti-takeover effect. Although not designed or intended for such purposes, the effect of the Authorized Shares Amendment might be to render more difficult or to discourage a merger, tender offer, proxy contest or change in control of us and the removal of management, which stockholders might otherwise deem favorable. For example, the authority of the Board to issue common stock might be used to create voting impediments or to frustrate an attempt by another person or entity to effect a takeover or otherwise gain control of us because the issuance of additional common stock would dilute the voting power of the common stock then outstanding. Our common stock could also be issued to purchasers who would support the Board in opposing a takeover bid which our Board determines not to be in our best interests and those of our stockholders. In addition to the Authorized Shares Amendment, the Certificate of Incorporation and Amended and Restated Bylaws also include other provisions that may have an anti-takeover effect. These provisions, among other things, permit the Board to issue preferred stock with rights senior to those of the common stock without any further vote or action by the stockholders, provide that special meetings of stockholders may only be called by the Board and some of our officers, and do not provide for cumulative voting rights, which could make it more difficult for stockholders to effect certain corporation actions and may delay or discourage a change in control. The Board is not presently aware of any attempt, or contemplated attempt, to acquire control of the Company and the Authorized Shares Amendment is not part of any plan by the Board to recommend or implement a series of anti-takeover measures.
Vote Required
Approval of this proposal requires the affirmative vote of a majority of the shares of our common stock outstanding on the record date for the Annual Meeting. Abstentions will have the same effect as an “against” vote on this proposal. As noted above, we believe that this proposal will be considered a “routine” matter and, as a result, we do not expect there to be any broker non-votes on this proposal. If, however, a broker non-vote occurs (or if your shares are not affirmatively voted in favor of this proposal for any other reason), it will have the same effect as an “against” vote on this proposal.
The Board of Directors Recommends
A Vote “For” Proposal 3.
Proposal 4
Approval of the Stock Option Exchange Program
On April 13, 2022, our Board authorized a stock option exchange program (the “Option Exchange”) pursuant to which our eligible employees would be given the opportunity to exchange eligible stock options for new stock options with an exercise price equal to the fair market value of our common stock at the time of the exchange. An eligible stock option generally includes any employee stock option that has an exercise price equal to or greater than $18.00 per share and was granted on or prior to December 31, 2021 pursuant to our 2018 Amended and Restated Equity Incentive Plan (the “2018 Plan”).
As of March 31, 2022, we had outstanding stock options held by employees to purchase 6,654,366 shares of common stock with a weighted average exercise price of $19.36 per share (exclusive of the Excluded Employees as defined below). Of these employee stock options, there were 4,070,873 shares with an exercise price equal to or greater than $18.00 per share with a weighted-average exercise price of $26.78 that would be considered eligible stock options for purposes of the Option Exchange.
The Board believes that the Option Exchange is in the best interests of stockholders and the Company, as new stock options granted under the program will provide added incentive to motivate and retain our talented employees.
Rationale for Option Exchange
We evaluated several alternatives for remaining competitive within our industry and with our employees, including increasing cash compensation and/or granting additional equity awards. While these components are part of our overall compensation packages, we do not believe that relying exclusively on such approaches is an ideal use of our resources. For example, increasing cash compensation would reduce the cash resources we devote to research and development, and granting additional stock awards would cause dilution to our current stockholders and greater expense. Accordingly, we determined that the Option Exchange was the most attractive alternative for stockholders for the reasons set forth below.
Employee Retention, Motivation and Performance
We have designed the Option Exchange to restore equity value, increase retention and motivation in a competitive labor market, provide non-cash compensation incentives and align our employee and stockholder interests for long-term value creation. Underwater stock option awards are of limited benefit in motivating and retaining our employees. Through the Option Exchange, we believe that we will be able to enhance long-term stockholder value by increasing our ability to retain experienced and talented employees and by aligning the interests of these individuals more fully with the interests of our stockholders.
Because 61% of our eligible employee stock options are underwater (and for a large number of employees, significantly so), as of March 31, 2022, we may face a considerable challenge in retaining our employees and there is a possibility that our competitors may be able to offer equity incentives that are more attractive, which in some cases, could make the terms of employment at a new employer more attractive than we can offer to our existing employees. Additionally, the underwater options have less perceived value and are unlikely to incentivize employees to work harder and build up value in the Company. The Option Exchange is designed to address these concerns, improve morale among our employees generally and reinvigorate a culture where equity compensation is a key component of our overall compensation package.
As discuss in more detail below, none of the new stock options issued under the Option Exchange program will be vested on the date of grant. Stock options issued in the Option Exchange will vest in equal annual amounts over a three-year period from the grant date of such new stock options. The stock options eligible to be exchanged are generally subject to a four-year vesting schedule, in which twenty-five percent of shares vest after one year, and the remaining shares vest in equal monthly installments. Our Compensation Committee believes that implementing a new extended vesting schedule is appropriate because it encourages retention of employees over the next three years, during a highly critical period for the Company.
Impact on Compensation Expense
The “fair value” of the stock options eligible for exchange was based on the then fair market value of our common stock on the applicable grant date. Under applicable accounting rules, we will recognize a total of approximately $73.9 million in non-cash compensation expense related to these underwater stock options, $36.3 million of which has already been expensed as of December 31, 2021 and $37.6 million of which we will continue to be obligated to expense, even if these stock options are never exercised because they remain underwater. Assuming that the exercise price of the replacement options will be equal to $9.11 per share (which was the fair market value of our stock on March 31, 2022), the replacement took effect on March 31, 2022 and all eligible employees participate, and that the inputs for the Black Scholes option pricing model used are held constant, the replacement options will result in an additional non-cash compensation expense of approximately $8.7 million. By replacing current stock options that have little or no
retention or incentive value with new stock options that will provide both retention and incentive value while being mindful of the additional compensation expense, we believe that we will be making efficient use of our resources.
Alternatives Considered
Our Compensation Committee considered alternatives to the Option Exchange to provide meaningful performance and retention incentive to our employees, including providing new equity to employees, repricing the options with no change in vesting, exchanging underwater options for full value shares, or increasing cash compensation. The Compensation Committee determined that the Option Exchange provides better performance and retention incentives with less cost to the Company or dilution to stockholders than the other alternatives.
Structure of the Option Exchange
The Board authorized the Option Exchange on April 13, 2022, subject to stockholder approval. It is currently anticipated that the Option Exchange will commence as soon as reasonably practicable following approval of this Proposal 4 by our stockholders, or the Commencement Date. At the start of the Option Exchange, employees holding eligible stock options will receive a written exchange offer that will set forth the precise terms of the Option Exchange. The written offer will be governed by the tender offer rules of the SEC. At or before the Commencement Date, we will file the offer to exchange and other related documents with the SEC as part of a tender offer statement on Schedule TO. Eligible optionholders will be given at least 20 business days to elect to participate in the Option Exchange. Eligible optionholders must choose to participate in the program for all of their eligible options or none, and may not choose to exchange portions of eligible option grants or some eligible option grants and not others. Set forth below is a descriptionbiographical information for each of the key features of the Option Exchange.
Eligible Participants
The Option Exchange will be available to employees, excluding our executive officers other than Dr. Chang, whose biographical information is set forth above.
Earl Douglas, 61, has served as the Company’s Senior Vice President, General Counsel and Compliance Officer since August 2023 and as our Corporate Secretary since January 2024. Before joining Allogene, Mr. Douglas served as Executive Vice President, General Counsel of Applied Molecular Transport. Prior to that role, he served in the same capacity for Kiverdi, Inc. He has also served as Vice President, General Counsel at BioMimetic Therapeutics, Spinal Dynamics, and OPX Biotechnologies. He previously served as Counsel with Wilson Sonsini Goodrich & Rosati, and earlier in his career practiced as an Associate with Weil, Gotshal & Manges. He earned his B.S. in chemical engineering from the Massachusetts Institute of Technology (MIT) and his J.D. from Columbia University School of Law.
Timothy Moore,62, has served as our Executive Vice President, Chief Technical Officer since April 2023. Previously, Mr. Moore served as the Chief Operating Officer of Instil Bio from September 2022 to December 2022, President and Chief CommunicationsOperating Officer (collectively,at PACT Pharma, Inc. from April 2020 to July 2022, and as the "Excluded Employees"), who on the Commencement Date are employed by usPresident and hold outstanding eligible stock options. AsChief Technology Officer at PACT from October 2019 to April 2020. Prior to PACT, Mr. Moore served as Executive Vice President, Technical Operations of Kite Pharma, or Kite, a Gilead Company, from March 31, 2022, eligible stock options were held by approximately 71%2016 to September 2019. Prior to Kite, he spent more than 12 years at Genentech, a Roche Company, most recently as Senior Vice President, Head of employees (exclusiveGlobal Technical Operations – Biologics and as a member of the Excluded Employees). ParticipantsGenentech Executive Committee. He holds a B.S. in Chemical Engineering from Tulsa University and an M.S. from Northwestern University.
Geoffrey Parker, 59, has served as our Executive Vice President, Chief Financial Officer since October 2023. Prior to joining us, Mr. Parker served as Chief Operating Officer, Chief Financial Officer and Executive Vice President of Tricida, Inc. Prior to joining Tricida, Mr. Parker served as Chief Financial Officer of Anacor Pharmaceuticals, and served as a Partner and Managing Director at Goldman Sachs, where he led the Option Exchange must continue to be employed by and provide services to us onWest Coast Healthcare Investment Banking group. In addition, Mr. Parker currently serves as a member of the date the surrendered options are cancelled and replacement stock options are granted. Any employee holding eligible stock options who elects to participate in the Option Exchange but whose service with us terminates for any reason before the date the new stock options are granted, includingboard of directors of Perrigo Company plc. He earned a termination due to voluntary resignation, retirement, involuntary termination, layoff, death or disability, would retain his or her eligible options subject to their existing terms and will not be eligible to receive new stock options in the Option Exchange.
Eligible Stock Options
An eligible stock option generally includes any eligible employee stock option that has an exercise price equal to or greater than $18.00 per share and was granted on or prior to December 31, 2021. The $18.00 price threshold represents an approximately 200% premium to the volume weighted average stock price for 30 trading days through April 13, 2022. As of March 31, 2022, we had outstanding eligible stock options to purchase 4,070,873 shares of common stock under the 2018 Plan at a weighted-average exercise price of $26.78 per share andB.A. with a weighted-average remaining lifedouble major in Economics and Engineering Sciences from Dartmouth College and an MBA from the Stanford Graduate School of 7.9 years. These eligible stock options represent approximately 2.8%Business.
Zachary Roberts, M.D., Ph.D.,46, has served as our Chief Medical Officer since April 2023 and as our Executive Vice President, Research and Development since January 2023. Previously, Dr. Roberts served as Chief Medical Officer for Instil Bio, Inc. (“Instil”) from March 2020 to November 2022. Prior to joining Instil, he served in various roles for Kite, during his four-year tenure, with his last position as Vice President, Clinical Development from February 2018 to May 2019. Prior to joining Kite, Dr. Roberts served in various roles in Amgen, with his last position as Clinical Research Medical Director for Amgen Oncology from January 2015 to July 2015. Dr. Roberts completed his training in internal medicine and hematology/oncology at the Massachusetts General Hospital and Dana Farber Cancer Institute. He earned his B.S. in microbiology and immunology from the University of Maryland, College Park and both his Ph.D. in immunology and his M.D. from the issued and outstanding sharesUniversity of our common stock as of March 31, 2022.Maryland, Baltimore.
Exchange Ratio
The Option Exchange is a one-for-one exchange. Thus, each eligible option will be replaced by a new option covering the same number of shares, but with a new exercise price, term, and vesting schedule. The overall number of stock options will stay the same.
Vesting Schedules for New Options
New stock option awards will not be vested on the date of grant. Eligible stock options may be exchanged for new stock options with a new three-year vesting schedule, in each case vesting in equal annual installments over the vesting term. These new vesting schedules support the nature of stock options as an incentive vehicle and provide us with valuable additional years of employee retention during a highly critical time for the Company.
Term for New Options
The new stock options will expire seven (7) years following the date the new options are granted.
Discussion of the Option Exchange As Soon As Practicable Following Stockholder ApprovalNamed Executive Officer Compensation
We expect that the Option Exchange will begin as soon as reasonably practicable, but no later than four (4) months following stockholder approval, if received. Our Board in its discretion reserves the right to amend, postpone or, under certain circumstances, cancel the Option Exchange once it has commenced, but the Option Exchange will not be materially amended inare a manner more beneficial to eligible participants without first seeking additional stockholder approval.
Impact of Option Exchange on Surrendered Options
Under the terms of the Option Exchange, there will be no shares returned to the share reserve of the 2018 Plan because it will be a one-for-one exchange.
Option Exchange Process
Additional information about how we expect to conduct the Option Exchange, if approved by stockholders, is set forth below. While the terms of the Option Exchange are expected to conform to the material terms described above in this Proposal 4, we may find it necessary or appropriate to change the terms of the Option Exchange to take into account our administrative needs, accounting rules, company policy decisions or to comply with any comments we receive from the SEC. We may decide not to implement the Option Exchange even if stockholder approval of the Option Exchange is obtained, or we may delay, amend or terminate the Option Exchange once it is in progress. The final terms of the Option Exchange will be described in the exchange offer documents that will be filed with the SEC and available at www.sec.gov.
Overview of the Option Exchange Process
Upon commencement of the Option Exchange, eligible participants holding eligible stock option awards will receive a written offer setting forth the terms of the Option Exchange and may voluntarily elect to participate. All eligible employees who are employed by us on the Commencement Date, are still employed by us on the grant date of the new stock options, and hold eligible stock option awards may participate in the Option Exchange. Eligible participants will be given at least 20 business days to elect to surrender eligible stock options in exchange for the same number of new stock options. Upon completion of the Option Exchange, surrendered stock options will be cancelled and new stock options will be granted. Cancelled options will then be available for future grantSmall Reporting Company under the 2018 Plan.
The 2018 Plan will govern all terms or conditions of new stock options not specifically addressed by the Option Exchange described in this proxy statement. Additionally, it is anticipated that new options will be incentive stock options (that is, they will qualify for the tax-favored treatment) to the extent allowable under Section 422 of the Internal Revenue Code and available for grant under our 2018 Plan.
Election to Participate
Eligible participants will receive a tender offer document and will be able to voluntarily elect to participate in the Option Exchange. If you are both a stockholder and employee holding stock options that are potentially subject to the Option Exchange, note that voting to approve the Option Exchange does not constitute an election to participate in the Option Exchange. The written exchange offer documents described above will be provided if and when the Option Exchange is commenced, and you can only elect to participate after that time.
Impact of Option Exchange on Number of Options Issued
The Compensation Committee established a 1-to-1 exchange ratio, that will result in the issuance of the same number of stock options through the Option Exchange.
Effect on Stockholders
Under the terms of the Option Exchange, the new stock options will have an exercise price commensurate with their fair market value at the time of the Option Exchange, making the stock options no longer underwater. As stated in the “Rationale for Option Exchange” Section, these new stock options should help keep our valuable employees at our Company and motivate them to create long-term value for our Company and stockholders.
Interests of Our Executive Officers and Non-Employee Directors in the Option Exchange
The Excluded Employees and our non-employee directors (including our Executive Chair) will not be permitted to participate in the Option Exchange.
Accounting Impact
The incremental compensation cost associated with the Option Exchange will be measured as the excess, if any, of the fair value of each award of new stock option granted to participants in the Option Exchange, measured as of the date the new stock options are granted, over the fair value of the stock options surrendered in exchange for the new stock options, measured immediately prior to the cancellation. This incremental compensation cost will be recognized ratably over the vesting period of the new stock options.
Material U.S. Federal Income Tax Consequences of the Option Exchange
The exchange of stock options pursuant to the Option Exchange should be treated as a non-taxable exchange because the new stock options will have an exercise price equal to or greater than the fair market value of our common stock on the grant date. Neither the Company, nor participants in the Option Exchange, should recognize any income for U.S. federal income tax purposes upon the grant of the new stock options. To the extent permissible and available for grant under our 2018 Plan, new stock options granted under the Option Exchange will be incentive stock options for U.S. federal income tax purposes. Tax effects may vary in other countries; a more detailed summary of tax considerations will be provided to all participants in the Option Exchange documents.
Financial Statements
Our consolidated financial statements and other information required by Item 13(a) of Schedule 14A under the Exchange Act are incorporated by reference from our Annual Report on Form 10-K for the fiscal year ended December 31, 2021, filed with the SEC on February 23, 2022.
Vote Required
Approval of the Option Exchange requires “For” votes from a majority of the shares present or represented by proxy at the Annual Meeting and entitled to vote on this proposal. Abstentions will have the same effect as an “Against” vote on this proposal. Broker non-votes will have no effect.
If you are both a stockholder and an employee holding eligible stock options, please note that voting to approve this program does not constitute an election to participate in the program.
Our Recommendation
The Board of Directors Recommends
A Vote “For” Proposal 4.
Proposal 5
Ratification of Selection of Independent Registered Public Accounting Firm
The Audit Committee of the Board of Directors has selected Ernst & Young LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2022 and has further directed that management submit the selection of its independent registered public accounting firm for ratification by the stockholders at the Annual Meeting. Ernst & Young LLP has audited the Company’s financial statements since its inception in 2017. Representatives of Ernst & Young LLP are expected to be present at the Annual Meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither the Company’s Bylaws nor other governing documents or law require stockholder ratification of the selection of Ernst & Young LLP as the Company’s independent registered public accounting firm. However, the Audit Committee of the Board is submitting the selection of Ernst & Young LLP to the stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, the Audit Committee of the Board will reconsider whether or not to retain that firm. Even if the selection is ratified, the Audit Committee of the Board in its discretion may direct the appointment of a different independent registered public accounting firm at any time during the year if they determine that such a change would be in the best interests of the Company and its stockholders.
The affirmative vote of the holders of a majority of the shares present at the Annual Meeting or represented by proxy and entitled to vote on the matter at the Annual Meeting will be required to ratify the selection of the accounting firm. Abstentions will have the same effect as an “against” vote on this proposal. Broker non-votes, if any, will have no effect.
Principal Accountant Fees and Services
The following table represents aggregate fees billed to the Company for the fiscal years ended December 31, 2020 and December 31, 2021 by Ernst & Young LLP, the Company’s principal accountant.
| | | | | | | | | | | |
| Fiscal Year Ended |
| 2021 | | 2020 |
| (in thousands) |
| Fee Category | | | |
| Audit fees(1) | $ | 1,580 | | $ | 1,331 |
| Audit-related fees | — | | — |
| Tax fees | — | | — |
| All other fees(2) | 2 | | 3 |
| Total fees | $ | 1,582 | | $ | 1,334 |
(1) Audit fees consist of fees for professional services provided primarily in connection with the annual audit of our financial statements, audit of our internal controls over financial reporting, quarterly reviews and services associated with SEC registration statements and other documents issued in connection with the Company’s at the market offerings, including comfort letters and consents.
(2) All other fees consist of a subscription to Ernst & Young Atlas Online, a proprietary knowledge management and research system.
All fees described above were pre-approved by the Audit Committee.
Pre-Approval Policies and Procedures.
Pursuant to its charter, the Audit Committee must review and approve, in advance, the scope and plans for the audits and the audit fees and approve in advance (or, where permitted under theapplicable rules and regulations of the SEC, subsequently) all non-audit services to be performed by the independent registered public accounting firm thatSecurities and Exchange Commission, and thus are not otherwise prohibited by law and any associated fees. The Audit Committee may delegate to one or more members of the committee the authority to pre-approve audit and permissible non-audit services, as long as this pre-approval is presented to the full committee at scheduled meetings.
The Board of Directors Recommends
A Vote “For” Proposal 5.
Proposal 6
Approval of an Adjournment of the Annual Meeting, if Necessary, to Solicit Additional Proxies
General
If the Annual Meeting is convened and a quorum is present, but there are not sufficient votes to approve Proposal 3, or if there are insufficient votes to constitute a quorum, our proxy holders may move to adjourn the Annual Meeting at that time in order to enable the Board to solicit additional proxies.
In this proposal, we are asking our stockholders to authorize the holder of any proxy solicited by the Board to vote in favor of adjourning the Annual Meeting to another time and place, if necessary or appropriate (as determined in good faith by the Board), to solicit additional proxies in the event there are not sufficient votes to approve Proposal 3. If our stockholders approve this proposal, we could adjourn the Annual Meeting and any adjourned or postponed session of the Annual Meeting and use the additional time to solicit additional proxies, including the solicitation of proxies from our stockholders that have previously voted. Among other things, approval of this proposal could mean that, even if we had received proxies representing a sufficient number of votes to defeat Proposal 3, we could adjourn the Annual Meeting without a vote on such proposal and seek to convince our stockholders to change their votes in favor of such proposal.
If it is necessary or appropriate (as determined in good faith by the Board) to adjourn the Annual Meeting, no notice of the adjourned meeting is required to be given to our stockholders, other than an announcement at the Annual Meeting of the time and place to which the Annual Meeting is adjourned, so long as the meeting is adjourned for 30 days or less and no new record date is fixed for the adjourned meeting. At the adjourned meeting, we may transact any business which might have been transacted at the original meeting.
Vote Required
Approval of this proposal requires the vote of the holders ofinclude a majority of the shares present at the Annual Meeting or represented by proxy and entitled to vote on the matter at the Annual Meeting. Abstentions will have the same effect as an “against” vote on this proposal. Broker non-votes, if any, will have no effect.
The Board of Directors Recommends
A Vote “For” Proposal 6.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding the ownership of the Company’s common stock as of March 31, 2022 by: (i) each director; (ii) each of the Company’s named executive officers; (iii) all executive officers and directors of the Company as a group; and (iv) all those known by the Company to be beneficial owners of more than 5% of its common stock.
The table is based upon information supplied by officers, directors and principal stockholders, Schedules 13D and 13G filed with the SEC and other sources believed to be reliable by the Company. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, the Company believes that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Applicable percentages are based on 143,569,902 shares outstanding on March 31, 2022, adjusted as required by rules promulgated by the SEC. The number of shares of common stock used to calculate the percentage ownership of each listed beneficial owner includes the shares of common stock underlying options or convertible securities held by such beneficial owner that are exercisable or convertible within 60 days following March 31, 2022. Unless otherwise indicated, the address for each person or entity listed in the table is c/o Allogene Therapeutics, Inc., 210 East Grand Avenue, South San Francisco, California 94080.
| | | | | | | | | | | | | | |
| Name of Beneficial Owner | | Number of shares Beneficially Owned | | Percentage Beneficially Owned |
| Greater than 5% Stockholders | | | | |
| Pfizer Inc.(1) | | 22,032,040 | | 15.3% |
| TPG GP A, LLC(2) | | 18,716,306 | | 13.0% |
| Directors and Named Executive Officers | | | | |
| David Bonderman(3) | | 18,716,306 | | 13.0% |
| Arie Belldegrun, M.D.(4) | | 8,858,860 | | 6.2% |
| David Chang, M.D., Ph.D.(5) | | 8,427,632 | | 5.9% |
| Joshua Kazam(6) | | 242,387 | | * |
| Franz Humer, Ph.D.(7) | | 314,966 | | * |
| Deborah Messemer(8) | | 287,215 | | * |
| Owen Witte, M.D.(9) | | 275,222 | | * |
| Elizabeth Barrett(10) | | 63,385 | | * |
| Vicki Sato, Ph.D.(11) | | 34,692 | | * |
| Todd Sisitsky | | — | | | — |
| John DeYoung | | — | | | — |
| Eric Schmidt, Ph.D.(12) | | 1,846,218 | | 1.3% |
| Alison Moore, Ph.D.(13) | | 1,680,278 | | * |
| Rafael Amado, M.D.(14) | | 764,418 | | * |
| Veer Bhavnagri(15) | | 1,017,493 | | * |
| All current executive officers and directors as a group (15 persons)(16) | | 42,529,072 | | 29.8% |
* Represents beneficial ownership of less than 1%.
(1) Consists of 22,032,040 shares of common stock held by Pfizer Inc. (“Pfizer”). The address of Pfizer is 235 E. 42nd Street, New York, NY 10017. This information is based on a Form 4 filed by PF Equity Holdings 2 B.V., a wholly-owned subsidiary of Pfizer, on April 4, 2022 with the SEC.
(2) Consists of an aggregate of 18,716,306 shares of common stock. TPG GP A is the managing member of TPG Group Holdings (SBS) Advisors, LLC, a Delaware limited liability company, which is the general partner of TPG Group Holdings (SBS), L.P., a Delaware limited partnership, which holds 100% of the shares of Class B common stock (which represents a majority of the combined voting power of the common stock) of TPG Inc., a Delaware corporation (“TPG”), which is the controlling shareholder of TPG GP Co, Inc., a Delaware corporation, which is the managing member of TPG Holdings I-A, LLC, a Delaware limited liability company, which is the general partner of TPG Operating Group I, L.P., a Delaware limited partnership, which is the sole member of each of (i) TPG GenPar VII Advisors, LLC, a Delaware limited liability company and (ii) The Rise Fund GenPar Advisors, LLC, a Delaware limited liability company. TPG GenPar VII Advisors, LLC is the general partner of TPG GenPar VII, L.P., a Delaware limited partnership, which is the general partner of TPG Carthage Holdings, L.P., a Delaware limited partnership, which directly holds 12,477,536 shares of Common Stock. The Rise Fund GenPar Advisors, LLC is the general partner of The Rise Fund GenPar, L.P., a Delaware limited partnership, which it the general partner of The
Rise Fund Carthage, L.P., a Delaware limited partnership (together with TPG Carthage Holdings, L.P., the “TPG Funds”), which directly holds 6,238,770 shares of Common Stock. Because of TPG GP A’s relationship with the TPG Funds, TPG GP A may be deemed to beneficially own the shares of Common Stock held by the TPG Funds. TPG GP A is owned by entities owned by Messrs. Bonderman, Coulter and Winkelried. Because of the relationship of Messrs. Bonderman, Coulter and Winkelried to TPG GP A, each of Messrs. Bonderman, Coulter and Winkelried may be deemed to beneficially own the shares of Common Stock held by the TPG Funds. Messrs. Bonderman, Coulter and Winkelried disclaim beneficial ownership of the shares of Common Stock held by the TPG Funds except to the extent of their pecuniary interest therein. The address of each of the TPG Funds is c/o TPG Global, LLC, 301 Commerce Street, Suite 3300, Fort Worth, Texas 76102. This information is based on the Schedule 13D/A filed on January 18, 2022 with the SEC.
(3) Consists of the shares described in note (2) above.
(4) Consists of (i) 4,710,120 shares of common stock beneficially owned by Rebecka Belldegrun, as beneficiary and trustee of the Bellco Legacy Trust fbo Rebecka Belldegrun, (ii) 195,039 shares of common stock beneficially owned by Bellco Legacy LLC, of which Dr. Belldegrun is a manager, (iii) 1,798,163 shares of common stock beneficially owned by Vida Ventures LLC (“Vida”), of which VV Manager LLC is the manager, of which Dr. Belldegrun is a Senior Managing Director, (iv) 302,849 shares of common stock held by Dr. Belldegrun, 173,739 of which will be subject to our right of repurchase as of 60 days of March 31, 2022, and (v) 1,852,689 shares of common stock issuable upon exercise of options,1,640,103 of which will be unvested but exercisable within 60 days of March 31, 2022 held by Dr. Belldegrun. Dr. Belldegrun disclaims beneficial ownership of the shares held by Vida, except to the extent of any pecuniary interest therein. The address of Dr. Belldegrun, Bellco Legacy Trust and Bellco Legacy LLC is 2049 Century Park East, Suite 1940 Los Angeles, CA 90067. The address of Vida is 40 Broad Street, #201, Boston, MA 02109.
(5) Consists of (i) 2,186,198 shares of common stock held by David Chang, M.D., Ph.D., 10,191 of which will be subject to our right of repurchase as of 60 days of March 31, 2022, (ii) 1,201,108 shares of common stock held by the Chang 2006 Family Trust (“Chang Trust”), (iii) 856,044 shares of common stock held by the JEC 2019 Trust, (iv) 856,044 shares of common stock held by the RTC 2019 Trust, and (v) 3,328,238 shares of common stock issuable upon exercise of options, 2,718,587 of which will be unvested but exercisable within 60 days of March 31, 2022. Dr. Chang is co-trustee of the Chang Trust, JEC 2019 Trust and RTC 2019 Trust.
(6) Consists of 242,387 shares of common stock held by Joshua Kazam.
(7) Consists of (i) 131,216 shares of common stock held by Franz Humer, Ph.D. and (ii) 183,750 shares of common stock issuable upon exercise of options.
(8) Consists of (i) 6,535 shares of common stock held by the Messemer Family Trust (“Messemer Trust”) and (ii) 280,680 shares of common stock issuable upon exercise of options, 19,836 of which will be unvested but exercisable within 60 days of March 31, 2022. Ms. Messemer is trustee of the Messemer Trust.
(9) Consists of (i) 221,182 shares of common stock held by Owen Witte, M.D., and (ii) 54,040 shares of common stock issuable upon exercise of options, 2,335 of which will be unvested but exercisable within 60 days of March 31, 2022.
(10) Consists of 63,385 shares of common stock issuable upon exercise of options.
(11) Consists of 34,692 shares of common stock issuable upon exercise of options.
(12) Consists of (i) 54,466 shares of common stock held by Eric Schmidt, Ph.D., (ii) 1,152,595 shares of common stock held by the Eric Schmidt 2017 Family Irrevocable Trust (“Schmidt Trust”), which were acquired by the Schmidt Trust upon the exercise of stock options held by Dr. Schmidt, 40,707 of which will be subject to our right of repurchase as of 60 days of March 31, 2022, and (iii) 639,157 shares of common stock issuable upon exercise of options, 491,455 of which will be unvested but exercisable within 60 days of March 31, 2022. Dr. Schmidt’s spouse is trustee of the Schmidt Trust.
(13) Consists of (i) 77,675 shares of common stock held by Alison Moore, Ph.D., and (ii) 1,602,603 shares of common stock issuable upon exercise of options, 888,831 of which will be unvested but exercisable within 60 days of March 31, 2022.
(14) Consists of (i) 33,234 shares of common stock held by Rafael Amado, M.D., and (ii) 731,184 shares of common stock issuable upon exercise of options, 537,793 of which will be unvested but exercisable within 60 days of March 31, 2022.
(15) Consists of (i) 371,589 shares of common stock held by Veer Bhavnagri, and (ii) 645,904 shares of common stock issuable upon exercise of options, 394,382 of which will be unvested but exercisable within 60 days of March 31, 2022.
(16) Includes the shares described in notes (3) through (15) above.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires the Company’s directors and executive officers, and persons who own more than ten percent of a registered class of the Company’s equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of the Company. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish the Company with copies of all Section 16(a) forms they file.
To the Company’s knowledge, based solely on a review of the copies of such reports furnished to the Company and written representations that no other reports were required, during the fiscal year ended December 31, 2021, all Section 16(a) filing requirements applicable to its officers, directors and greater than ten percent beneficial owners were complied with, other than one late Form 4 filing reporting two transactions for Dr. Chang.
Executive Compensation
Compensation Discussion and Analysis in this Proxy Statement. However, we believe providing additional disclosure regarding our compensation philosophy, policies and principles underlying our executive compensation decisions constitutes good corporate governance and provides our stockholders with valuable information regarding our executive compensation practices. In particular, this Discussion of Named Executive Officer Compensation is designed to provide you with a better understanding of our compensation philosophy and how our Compensation Committee makes decisions regarding compensation for our named executive officers listed below.
Overview
This Compensation Discussion and Analysisof Named Executive Officer Compensation discusses the compensation philosophy, policies and principles underlying our executive compensation decisions for 2021.2023. It provides qualitative information on the factors relevant to these decisions and the manner in which compensation is awarded to our named executive officers (our “Named Executive Officers” or “NEOs”) for the fiscal year ended December 31, 2021,2023, which consist of our principal executive officer principal financial officer and our remaining threethe Company’s two other most highly compensated executive officers during 2021.2023. Our Named Executive Officers for 20212023 were:
•David Chang, M.D., Ph.D., our President and Chief Executive Officer;
•Eric Schmidt, Ph.D.,Timothy Moore, our Executive Vice President, Chief FinancialTechnical Officer; and
•Rafael Amado,Zachary Roberts, M.D., Ph.D. our Chief Medical Officer and Executive Vice President, Research and Development;Development and Chief Medical Officer.
•Alison Moore, Ph.D.,
Corporate Highlights for 2023
The Compensation Committee, in consultation with the Board, is responsible for establishing, implementing, and overseeing our Chief Technical Officer;overall compensation strategy and
•Veer Bhavnagri, policies, including our General Counsel.
Executive Summary
2021 Business Highlights. During 2021, we achieved several importantexecutive compensation program, in a manner that supports our business milestones, including, but not limitedobjectives. Our Compensation Committee determined that in 2023 the Company continued to the following:
•Advanced our innovative pipeline:
o Continued enrollment in the Phase 1 clinical trial ("ALPHA trial") of ALLO-501 in patients with relapsed/refractory non-Hodgkin lymphoma (“NHL”), the Phase 1/2 clinical trial ("ALPHA2 trial") of ALLO-501A in patients with relapsed/refractory NHL, and in the Phase 1 clinical trial ("UNIVERSAL trial") of ALLO-715 in patients with relapsed/refractory multiple myeloma. In addition, in the first half of 2021, we expanded the UNIVERSAL trial to assess ALLO-715 in combination with SpringWorks Therapeutics, Inc.’s investigational gamma secretase inhibitor, nirogacestat.
o Reported interim data for the ALPHA, ALPHA2 and UNIVERSAL trials at the American Society of Hematology ("ASH") annual meeting in December 2021, providing continued proof-of-concept ofadvance its allogeneicchimeric antigen receptor (“CAR”(“CAR”)T cell therapy in blood cancers.
o Advanceddevelopment programs, however 2023 became a transition year with respect to both our anti-CD70 CAR T cell product candidate, ALLO-316, that may have potential for various hematologic and solid tumor indications. In the first half of 2021, we initiated a Phase 1 clinical trial of ALLO-316 in patients with advanced or metastatic clear cell renal cell carcinoma.
o Progressed our technology platform that we call “TurboCARs” to mimic cytokine activation signaling within a CAR T cell, which could enhance the proliferative potential, migratory behavior, and killing activity of cells. In mid-2021, we initiated a Phase 1 clinical trial of our first TurboCAR, ALLO-605, in patients with relapsed/refractory multiple myeloma.
o Obtained FDA designations for advancing thekey development or any commercialization of our product candidates, including Fast-Track designation for ALLO-605, Regenerative Medicine Advanced Therapy designation for ALLO-715 and Orphan Drug Designation for ALLO-715.
•Further built-out our capabilities:
o Advanced Allogene Overland Biopharm (CY) Limited, our joint venture with Overland Pharmaceuticals (CY) Inc. for the development, manufacturing and commercialization of certain of our product candidates targeting BCMA, CD70, FLT3, and DLL3 in China, Taiwan, South Korea and Singapore. In 2021, we hired a new chief executive officer, leased a facility for manufacturing and began technology transfer for manufacturing.
o Initiated GMP manufacturing from our cell therapy manufacturing facility in Newark, California.
o Entered into a lease agreement to expand our headquarters in South San Francisco, California. The lease is expected to commence on April 1, 2022, and the additional 47,566 square feet of office and laboratory space will support the growth of our research platform and correlative clinical development activities.
o Strengthened our board of directors with the appointment of Ms. Barrett and Dr. Sato,programs and our scientific advisory board withexecutive leadership team.
As the appointment of Jae Park, M.D.
o Grew our organization from 264 employeesCompensation Committee performed its annual compensation review at the beginning of 2023, the year to 301 employees at the endCommittee was particularly concerned with retention of the year.
In addition to the above achievements, we overcame a significant unexpected regulatory challenge. In October 2021, the FDA placed a hold onexecutive team, particularly our clinical trials. The clinical hold followedCEO. One executive officer, our notification to the FDA of a chromosomal abnormalityExecutive Vice President, Research and Development, had resigned in an ALPHA2 trial patient which was detected in a bone marrow biopsy undertaken to assess pancytopenia. Investigations concluded that the chromosomal abnormality was unrelated to TALEN gene editing or our manufacturing processDecember 2022, and had no clinical significance. The investigation also determined that the abnormality was not detected in any of our manufactured product candidates or in any other patient treatedthese concerns materialized with the same ALLO-501A lot. The abnormality occurred in the patient after the cell product was administered and involved regionsbroader turnover of the T cell receptor and immunoglobulin genes known to undergo rearrangement as part of the T cell or B cell maturation process. The FDA found that we satisfactorily addressed all clinical hold issues and removed the hold in January 2022. The Compensation Committee and the Board refrained from rewarding any additional compensation during the clinical hold and approved the 2021 annual incentive bonus and 2022 base salary adjustments after the clinical hold was resolved in January 2022.
We believe the achievements highlighted above as well as resolving the clinical hold were a result of exceptional strategic, technical and operational leadership.
2021 Executive Compensation Policies and Practices. During 2021, our executive compensation policies and practices included the following:
•Compensation Committee of Independent Directors. The Compensation Committee is composed of all independent directors and includes our lead independent director.
•Annual Compensation Review. The Compensation Committee undertakes a comprehensive review of compensation of our senior vice presidents and above, including our Named Executive Officers, on an annual basis.
•Independent Compensation Consultant. The Compensation Committee engages its own compensation consultant, and reviews its independence from management.
•Risk Analysis. We review the structure of our executive compensation program to minimize the risk of inappropriate risk-taking by our executive officers.
•No Guaranteed Compensation. Although we have signed employment agreements with each of our Named Executive Officers, these agreements provide for “at will” employment, and none of these agreements provides any guarantees relating to base salary increases or the amounts of any annual incentive awards or long-term equity awards.
•Multi-Year Vesting. The equity awards granted to our executive officers generally vest over multi-year periods, consistent with current market practice and our retention objectives.
•No Special Retirement Benefits. We do not provide pension arrangements or post-retirement health coverage for our executive officers or employees. Our executive officers and other U.S.-based employees are eligible to participate in our Section 401(k) plan, which is a retirement savings defined contribution plan established in accordance with Section 401(a) of the Internal Revenue Code of 1986 (the “Code”). We currently make matching contributions into the Section 401(k) plan on behalf of participants. We match 100% of eligible contributions up to the first 3% of eligible compensation, with an additional match of 50% on the next 3% (maximum of 4.5%).
•No Special Health or Welfare Benefits or Perquisites. Our executive officers participate in broad-based company-sponsored health and welfare benefits programs on the same basis as our other full-time, salaried employees. We generally do not provide perquisites or other personal benefits to our executive officers, other than those we provide to our employees generally.
•No Tax Reimbursements. We do not provide any tax reimbursement payments (including “gross-ups”) on any perquisites or other personal benefits.
•Policy Against Hedging and Speculative Trading and Pledging our Common Stock. Our insider trading policy prohibits our employees from engaging in “hedging” or other inherently speculative transactions with respect to our common stock or borrowing against our common stock. Specifically, no officer, director, other employee or consultant may engage in short sales, transactions in put or call options, hedging transactions or other inherently speculative transactions with respect to our
common stock at any time. In addition, no officer, director, other employee or consultant may margin, or make any offer to margin, or otherwise pledge as security, any of our common stock, including without limitation, borrowing against such stock, at any time.
•Stock Ownership Guidelines Policy.CEO, during 2023. The purpose of our Stock Ownership Guidelines Policy is to encourage ownership of our common stock, promote the alignmentCompensation Committee was of the long-term interests of our non-employee directors, Chief Executive Officer, and other executive officers with the long-term interests of our stockholders, and further promote our commitment to sound corporate governance. Under the Guidelines, the target stock ownership level for our President and Chief Executive Officer is six times (6x) his base annual salary, the target stock ownership level for our non-employee directors is five times (5x) their base annual cash retainer and the target stock ownership level for our other executive officers is one times (1x) their base annual salary. Under these Guidelines, the compliance deadline for all of our executive officers and directors is the later of December 2023 and five years from an individual's commencement of employment or appointment to our Board, although we expectview that the target stock ownership levels likely willoutstanding equity grants held by the executive team would not be achieved much sooner than that.
2021 Executive Compensation Highlights. Despite the challenge of the clinical hold, we made significant progress towards critical clinical, research, manufacturing, and other strategic milestones,effective as described above. With respect to 2021 compensation decisions, the Compensation Committee and our Board focused on ensuring thata retention tool or as performance incentives, because a significant portion of the total directoutstanding stock grants were “underwater, ” meaning that the stock option exercise price exceeded the market price of our common stock. As such, the Compensation Committee was concerned that the outstanding stock option grants would provide little or no perceived value to the option holders. In an effort to balance stockholders’ general desire for a performance-oriented compensation awardedstructure with the need to incentivize and retain the executive team, the Compensation Committee added performance stock units (“PSUs”) as part of its annual equity grants for 2023. See the discussion below under heading “Executive Compensation—Executive Compensation Program and Compensation Decisions for the Named Executive Officers—Long-Term Incentive Compensation”).
Despite the transitions in our management team, throughout 2023 we continued to execute the Company’s most advanced clinical trial programs, ALPHA2 and EXPAND. In late 2023, the work done to advance our lead product candidate, cemacabtagene ansegedleucel (“cema-cel,” previously ALLO-501A) into a first line (“1L”) consolidation trial (“ALPHA3”) came to fruition and accordingly, afforded the management team the opportunity to reassess the Company’s development strategy to make the long-term commercial prospect more competitive. The management team recognized that the outcome of ALPHA3 could allow cema-cel to be embedded in the 1L setting as a consolidation treatment to boost cure rates. We believe this may redefine the future of CAR T therapy and potentially reposition our allogeneic CAR T
product as the only CAR T to be part of a 1L treatment plan for newly diagnosed and treated large B-cell lymphoma (“LBCL”) patients. This could greatly enhance cema-cel’s market opportunity by competitively placing use of cema-cel ahead of other CAR T therapies and potentially rendering later-line treatment (including those being investigated in ALPHA2 and EXPAND) obsolete. As a result, the Company made the decision to deprioritize ALPHA2 and EXPAND in the third line (“3L”) setting, and focus on ALPHA3.
Following our 2023 reprioritization, earlier this year we announced our 2024 Platform Vision, under which we are focusing on four core programs:
•Large B-Cell Lymphoma (LBCL): Potentially groundbreaking ALPHA3 Trial that we believe may leapfrog other CAR T’s and embed cema-cel in 1L LBCL treatment in community cancer centers where most newly diagnosed patients seek care.
•Chronic Lymphocytic Leukemia (CLL): New Phase 1 ALPHA2 Cohort is designed to evaluate cema-cel as a CLL treatment to address the limitations of autologous therapies in a disease where poor T cell fitness is a known barrier to efficacy.
•Autoimmune Disease (AID): ALLO-329, our next-generation CD19 Dagger® program, focuses on scalability and reduced or chemotherapy-free lymphodepletion, positioning allogeneic CAR T to potentially transform autoimmune management and meet the demand of the market.
•Renal Cell Carcinoma (RCC): Ongoing TRAVERSE trial with ALLO-316 seeks to advance scientific innovation underlying the Dagger® technology to optimize CAR T cell expansion and persistence, thereby maximizing the potential of allogeneic CAR T in solid tumors while mitigating treatment-associated inflammatory response.
Compensation Highlights for 2023
In view of the deprioritization of ALPHA2 and EXPAND, a large percentage of the Company’s corporate goals for 2023 could not be fully achieved. Because our Compensation Committee adheres to a longstanding pay-for-performance philosophy, the failure to fully achieve such goals is reflected in the Compensation Committee’s relatively low scoring of the corporate goals resulting in an achievement level of 57.5%. A general description of the 2023 milestones in support of the Company’s business strategy and this conclusion by the Compensation Committee are described below under the heading “Annual Cash Incentive Plan.”
The Compensation Committee evaluates our compensation program, taking into consideration best practices and emerging trends, stockholder input as well as data and feedback provided by our independent executive compensation consultant, Compensia. In the past year, we have continued to take measures to align our compensation program with stockholder interests including the following actions:
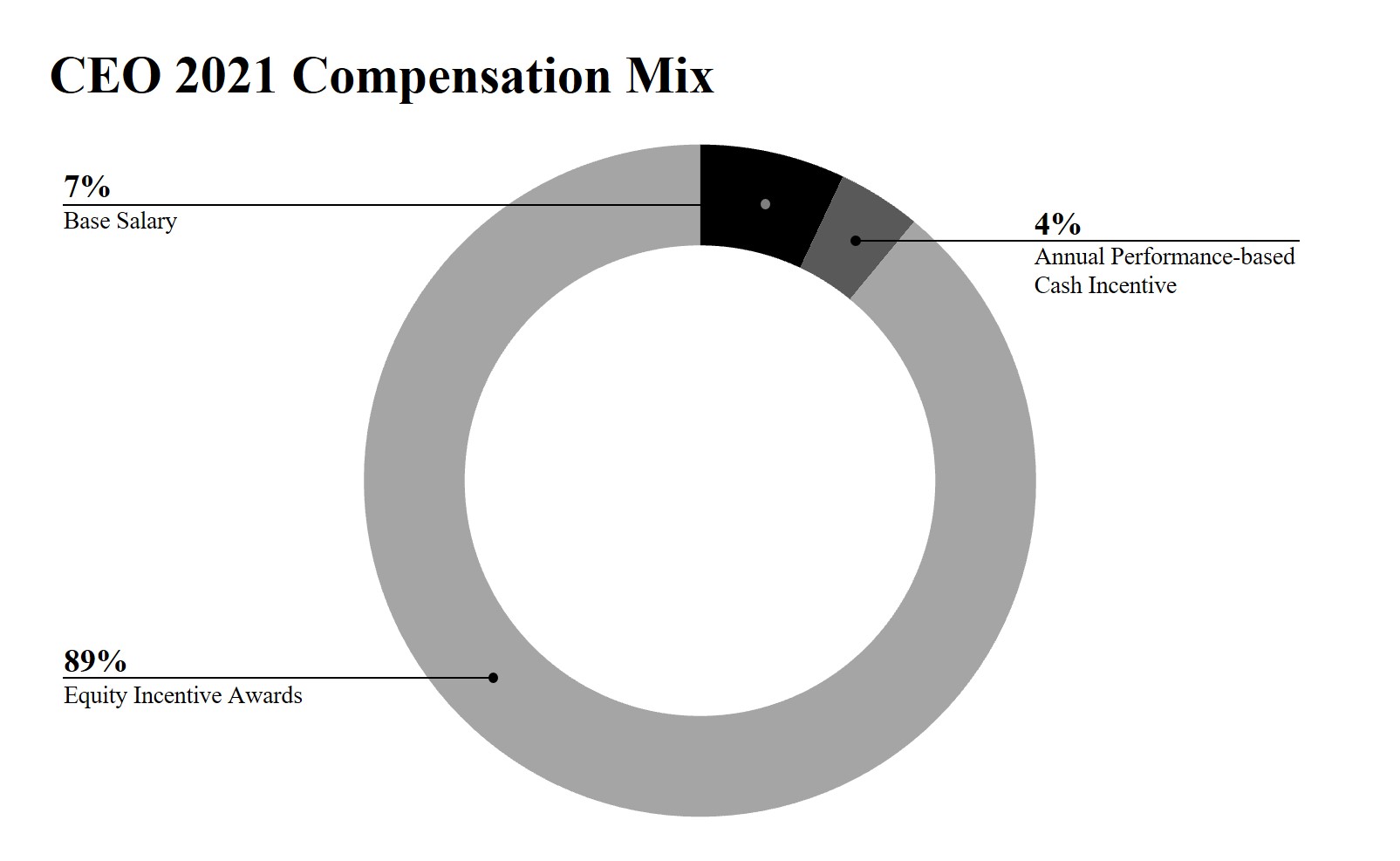
•In 2023, excluding Dr. Chang, our Named Executive Officers was performance-based and linked to meeting our long-term strategic plan to create long-term stockholder value.Officer’s base salaries comprised approximately 6% of their total compensation, on an aggregate basis. Dr. Chang’s 2023 base salary represented approximately 5% of his total compensation.
The substantial
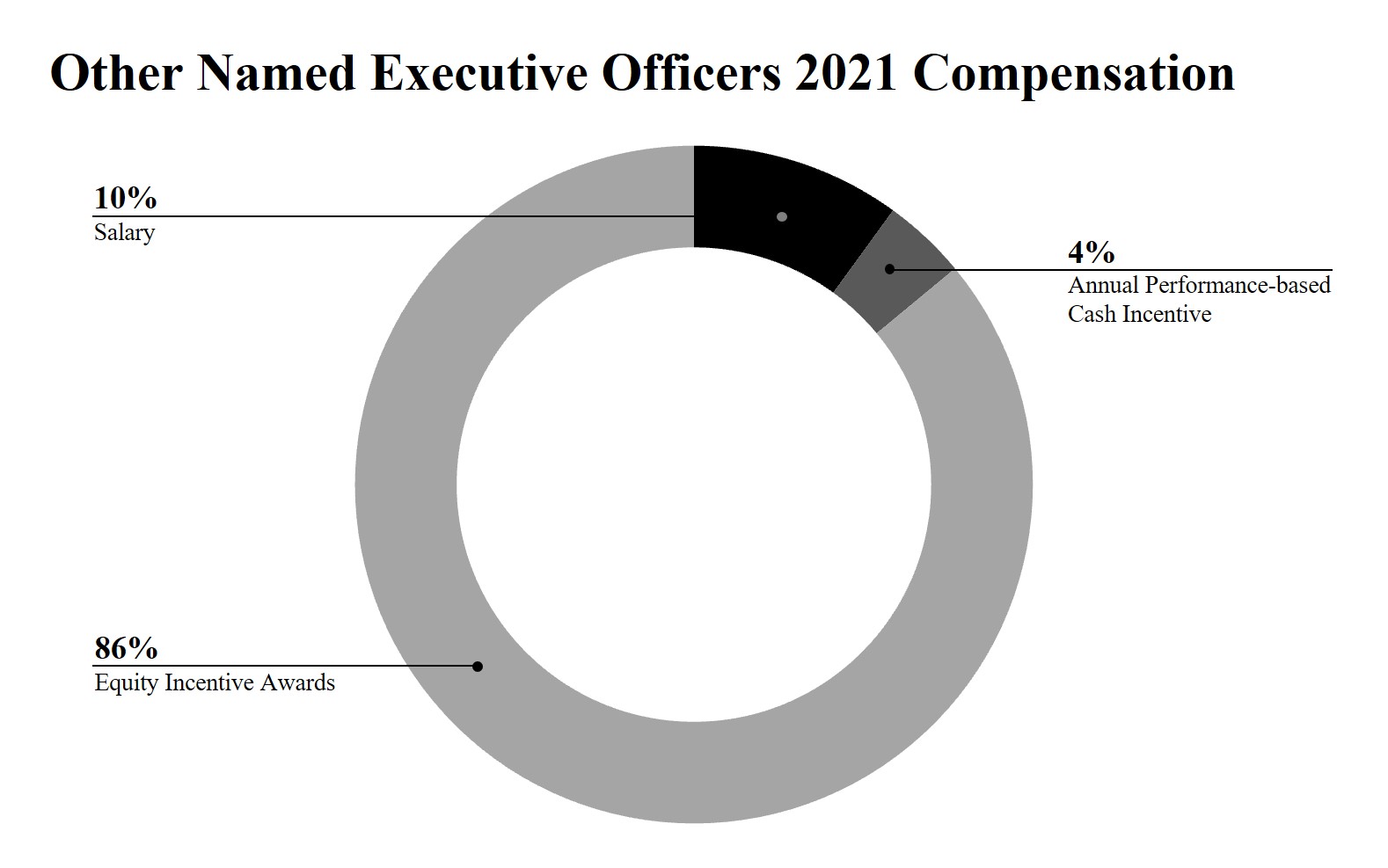
•A large majority of our 2021 compensation to Named Executive OfficersOfficers’ compensation was represented by long-term incentives, which are inherently performance based. Approximately 93% of Dr. Chang’s total compensation for 2023 was in the form of equity incentive awards. We believe that equity incentive awards further our long-term strategic plan to create long-term stockholder value. In recognition of our significant achievements and as a retention measure, the Compensation Committee also granted 2021 annual equity awards to Dr. Schmidt, Dr. Amado, Dr. Moore and Mr. Bhavnagri and our Board granted 2021 annual equity awards to Dr. Chang. The following charts illustrate the portion of compensation attributable to equity incentive awards, annual performance-based cash incentive awards and base salary for our Chief Executive Officer and forincentives. For our other Named Executive Officers, on average, approximately 91% of their total compensation for 2023 was represented by long-term incentives.
•For 2023 annual long-term incentives, in March 2023 the Compensation Committee determined that Dr. Chang, along with all the executive officers serving at that time, except for Dr. Roberts, would receive 35% of the target dollar value in stock options, 15% in restricted stock units (“RSUs”), and 50% in performance stock units (“PSUs”). As described in more detail below, Dr. Roberts was given a pro-rated PSU award since he joined the Company in January 2023.

The graphs above display the base salary, cash incentive, long-term incentives (stock option grant date fair value, RSU grant date fair value and PSU grant date fair value) of our Chief Executive Officer, Dr. Chang (left chart) and other NEOs (Dr. Roberts and Mr. Moore) (right chart). Because Dr. Roberts and Mr. Moore both started with the Company in 2023, their compensation mix shown in the chart above includes new hire grants, sign-on bonuses and other compensation, each as reported in the Summary Compensation Table. As a result, their equity compensation mix differs significantly from our CEO’s equity compensation mix as reflected in the charts above. The target dollar value used by the Compensation Committee in establishing market-based PSU grants (i.e., PSUs wherein the vesting performance measure is based on achieving a target Allogene stock price) differs from the grant date fair value calculated under FASB ASC Topic 718 in which the value is discounted using the Monte Carlo simulation. The values reflected in the graphs above and the compensation tables below reflect the grant date fair values.
Executive Compensation Governance Practices
Below we summarize certain executive compensation related governance practices that we follow and that we believe serve our stockholders’ long-term interests.
| | | | | |
| WHAT WE DO |
| ✓ | DO maintain an executive compensation program designed to align pay with performance |
| ✓ | DO conduct an annual say on pay advisory vote |
| ✓ | DOalign pay and performance with a significant percentage of total compensation linked to the achievement of Company operational and strategic goals established at the beginning of the performance period by the Compensation Committee, and in the case of NEOs other than the CEO, individual performance, and contribution in achieving the Company goals |
| ✓ | DO maintain a Compensation Committee comprised solely of independent directors |
| ✓ | DOhave double trigger on executive change in control severance arrangements |
| ✓ | DOprohibit speculative trading of company stock (short sales, put or call options, hedging transactions, etc.) |
| ✓ | DO prohibit pledging or the use of common stock to secure a margin or other loan |
| ✓ | DO retain an independent compensation consultant |
| ✓ | DOmaintain stock ownership guidelines for executives and directors |
| ✓ | DOmaintain an executive officer recoupment (“claw back”) policy |
| ✓ | DOconduct an annual comprehensive compensation program risk assessment |
| ✓ | DO evaluate officer compensation levels against a peer group of similarly situated companies |
| ✓ | DO annual comprehensive review of compensation of our senior vice presidents and above, including our NEOs |
| ✓ | DOrequire equity awards granted to our executive officers vest over multi-year periods or vest based on the achievement of key strategic goals |
| | | | | |
| WHAT WE DO NOT DO |
| ✘ | NO incentives that may motivate excessive risk-taking or present a windfall risk |
| ✘ | NO pay decisions that circumvent pay-for-performance, such as options backdating or waiving performance requirements for performance stock units |
| ✘ | NO repricing or replacing of underwater stock options/SARs without prior shareholder approval (including cash buyouts and voluntary surrender of underwater options) |
| ✘ | NO agreements providing for tax gross-ups for any employee or director |
| ✘ | NO material perquisites |
| ✘ | NO excessive termination or CIC severance payments (2 years or less) |
| ✘ | NO executive single-trigger change in control benefits |
| ✘ | NO severance payments or benefits for executives who voluntarily terminate their employment |
| ✘ | NOguaranteed compensation and all executives are employed “at will” |
| ✘ | NO guaranteed annual bonus payouts |
| ✘ | NO benefits for our executives not generally available for all employees |
| ✘ | NO granting of in-the-money stock options |
“Say on Pay” Consideration
At our 2023 annual meeting of stockholders, approximately 68.4% of the shares voted at the meeting (exclusive of broker non-votes) were voted in favor of the approval, on an advisory basis, of the compensation of our named executive officers as disclosed in the proxy statement for that annual meeting. In light of the prior year’s say on pay results (93% in June 2022), we were disappointed by the 2023 results. The Compensation Committee considered this support carefully, and took the following meaningful steps in direct response to the 2023 say on pay voting results:
•Significantly reduced the grant date fair value of the CEO’s and Executive Chair’s January 2024 annual equity grants to approximately 26% of the 2023 grant date fair value.
•Implemented no base salary increase in 2024 for the CEO and the Executive Chair (for the second consecutive year) and approved only a modest adjustment (1%) for one of the remaining NEOs to bring his base compensation in line with approximately the 50th percentile of our peer group.
•The level of stockholder support was also a significant factor in the Company’s decision to voluntarily provide the additional disclosure included in this Discussion of Named Executive Officer Compensation to ensure greater transparency, although not required as a group.Smaller Reporting Company, which includes:
◦More detailed disclosure regarding how the Compensation Committee, in consultation with its independent compensation consultant, set the Executive Chair’s compensation (see disclosure below under the heading “Executive Compensation—Executive Chair Compensation”), and more detailed disclosure regarding the Executive Chair’s role, responsibilities and contributions to the Company which we believe provide a compelling rationale in support of his compensation (see disclosure above under the heading “Information Regarding the Board of Directors and Corporate Governance—Board Leadership Structure”); and
◦Detailed disclosure regarding the 2023 equity grants, which included a mix of options, RSUs and PSUs, and disclosure regarding how the Compensation Committee used PSUs with aggressive vesting events (over 350% increase in share price and product regulatory approval targets) in order to directly align executive compensation with creating long-term stockholder value (see disclosure below under the heading “Executive Compensation Program and Compensation Decisions for the Named Executive Officer—Long-Term Incentive Compensation”).
In addition, the Compensation Committee considered that the absence of performance-based equity awards in our 2022 executive compensation programs as described in last year’s proxy statement may have been a significant consideration for those stockholders who did not support last year’s say on pay. The Compensation Committee believes the introduction of PSUs during 2023, in addition to stock options and RSUs, addresses this concern. As discussed in greater detail below,
in structuring the Company’s 2023 equity grants to executives, the Compensation Committee sought to balance critical retention needs with the performance-based expectations of investors while focusing management on the creation of long-term shareholder value. This was done through the inclusion of PSUs in the equity mix granted for 2023, in addition to stock options, both of which the Compensation Committee views as performance-oriented, and RSUs which the Committee views as geared more towards retention.
The Compensation Committee will monitor and continually evaluate our compensation program going forward in light of our stockholders’ views and our transforming business needs. Our Compensation Committee expects to continue to consider the outcome of our say on pay votes and our stockholders’ views when making future compensation decisions for our NEOs.
Executive Compensation Philosophy and Overview
We are a clinical-stage biotechnology company pioneering the development of allogeneic CAR T cell (AlloCAR T™) products for cancer and autoimmune disease. We operate in a very technical, highly regulated, and extremely competitive industry where our long-term success is highly dependent on the specialized skills, talent, and dedication of our executive officers. Our Compensation Committee believes that a well-designed compensation program should align executive interests with the drivers of growth and stockholder return, while enabling us to attract and retain employees whose talents, expertise, leadership, and contributions are critical to building and sustaining growth in long-term stockholder value. As a result, we utilize a strong pay-for-performance compensation philosophy, with total compensation levels for our executive officers, including our NEOs, correlated to the achievement of corporate goals and individual performance, and calibrated to our peers with whom we compete for talent.
Our executive compensation program is intended to meet five principal objectives:
•Enable us to attract, retain and motivate superior talent;
•Link rewards to the achievement of critical strategic priorities;
•Create incentives for our executive officers to further our long-term strategic plan to create long-term stockholder value;
•Provide appropriate levels of risk and reward relative to an executive officer's position with us; and
•Differentiate compensation based on individual performance.
Based on this philosophy, our performance-driven compensation program primarily consists of three components: base salary, annual cash bonusincentive opportunity, and long-term incentive compensation in the form of equity awards. The Compensation Committee has determined that these three components, with a portionthe vast majority of target total direct compensation allocated to “at-risk” performance-based incentives through the use of short-term and long-term incentive compensation, best align the interests of our executive officers with those of our stockholders. While it does not have any formal policies for allocating compensation among the three components, the
Compensation Committee reviews relevant competitive market data and uses its judgment to determine the appropriate level and mix of compensation on an annual basis to ensure that compensation levels and opportunities are competitive and that we are able to attract and retain capable executive officers to work for our long-term prosperity and stockholder value, without taking unnecessary or excessive risks.
2023 Summary Compensation Table
The following table sets forth all of the compensation awarded to, earned by or paid to our Named Executive Officers during the fiscal years ended December 31, 2023, December 31, 2022 and December 31, 2021.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and principal position | | Year | | Salary ($) | | Bonus ($)(1) | | Stock awards ($)(2) | | Option awards ($)(3) | | Non-equity incentive plan compensation ($)(4) | | All other compensation ($)(5) | | Total ($) |
| David Chang, M.D., Ph.D. | | 2023 | | 724,000 | | — | | 7,996,888 | | 5,070,365 | | 270,595 | | — | | 14,061,848 |
| President and Chief Executive Officer | | 2022 | | 724,000 | | — | | — | | 13,697,357 | | 376,480 | | — | | 14,797,837 |
| | 2021 | | 695,000 | | — | | 2,717,669 | | 6,352,635 | | 396,150 | | — | | 10,161,454 |
Timothy Moore Chief Technology Officer | | 2023 | | 360,938 | | 100,000 | | 2,249,987 | | 2,999,886 | | 93,416 | | — | | 5,804,227 |
Zachary J. Roberts M.D., Ph.D. Chief Medical Officer and Executive Vice President of Research and Development | | 2023 | | 522,813 | | 75,000 | | 3,533,710 | | 3,850,445 | | 134,727 | | 14,850 | | 8,131,545 |
(1) The dollar amounts in this column reflect the sign-on bonuses paid to Dr. Roberts and Mr. Moore in 2023.
(2) The dollar amounts in this column reflect the aggregate grant date fair value of RSUs and PSUs granted during the indicated year and computed in accordance with FASB ASC 718. The grant date fair value of the 2023 RSUs is based on the closing market price of the Company’s common stock on the date of grant. The grant date fair value of the 2023 PSUs is determined using Monte Carlo simulation for PSUs that vest upon achievement of a market condition. For PSUs that vest upon achievement of a performance condition, the grant date fair value is based upon the probable outcome of such condition. Assuming that maximum performance is achieved, the value of the PSUs with performance conditions granted to Dr. Chang, Mr. Moor and Dr. Roberts in 2023 would have been $3,730,502, $1,249,991 and $1,749,858, respectively, at the date of grant under FASB ASC 718. For a discussion of valuation assumptions, see Note 10 “Stock-based Compensation” to our financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2023.
(3) The dollar amounts in this column represent the aggregate grant date fair value of stock option awards granted during the indicated year. These amounts have been computed in accordance with FASB ASC Topic 718, using the Black-Scholes option pricing model. For a discussion of valuation assumptions, see Note 10 “Stock-based Compensation” to our financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2023. These amounts do not reflect the actual economic value that will be realized by the Named Executive Officer upon the vesting of the stock options, the exercise of the stock options, or the sale of the common stock underlying such stock options.
(4) The dollar amounts in this column represent annual performance-based incentive earned for the indicated year. For more information, see above under “2023 Performance-Based Cash Incentive.”
(5) The dollar amounts in this column represent Section 401(k) Company contributions for Dr. Roberts in 2023.
Narrative to Summary Compensation Table
Process for Setting Executive Compensation
We seek to foster a performance-oriented culture, where individual performance is aligned with organizational objectives. To achieve this, we evaluate and reward our executive officers based on their contributions to the achievement of annual goals and objectives set early in the year. Performance is reviewed at least annually through processes discussed further below, with a focus on our research, clinical, regulatory, financial and operational performance, and in view of economic and financial conditions affecting the performance period. In addition, the Compensation CommitteeWe also evaluate and our Board considered the positive results of the shareholder advisory vote onadjust executive compensation held in 2021, where 69% of shareholders voted in favor of, and concludedresponse to our approach to compensation for named executive officers was appropriate.say on pay results from our annual stockholder meetings.
Role of the Compensation Committee
The Compensation Committee reviews and approves our executive compensation philosophy, objectives and methods, evaluates our performance and the performance of our executive officers, and either approves executive compensation or
makes recommendations for ratification by our Board members. Please see “Information Regarding Committees of ourthe Board of Directors—Compensation Committee—Compensation Committee Processes and Procedures” for additional information.
Awards of performance-based compensation for the previous year are typically made at the last-scheduled Compensation Committee meeting of the year or first-scheduled meeting of the following year. Adjustments to the base salaries of our Named Executive Officers, if any, are also typically made at the last-scheduled Compensation Committee meeting of the year. Due to the clinical hold in the fourth quarter of 2021, our Board and Compensation Committee deferred year-end compensation matters until after resolutionyear or first-scheduled meeting of the clinical hold in January 2022.following year. The Compensation Committee typically reviews annual equity awards in the first quarter of the year.
Role of Management
In making compensation decisions, the Compensation Committee considers the recommendations of our Chief Executive Officer, with input from our Executive Chair, Chief People Officer and General Counsel and Secretary.Counsel. Our Chief Executive Officer, Dr. Chang, makes recommendations to the Compensation Committee with respect to our executive officers, but is not present and does not participate in the deliberations or determination of his own compensation. The Compensation Committee may review and approve the corporate objectives and goals pursuant to the powers delegated under its charter. Dr. Chang annually leads the development of our corporate objectives and goals, which are typically reviewed and approved by the Compensation Committee and then our Board. Dr. Chang provided our business and operations perspective for the Compensation Committee’s final review of progress made on the goals set for 2021.2023. Other than as described above, no other executive officers participate in the determination or recommendation of the amount or form of executive officer compensation.
Role of Independent Compensation Consultant
The Compensation Committee is authorized to engage a compensation consultant or other advisors to review our executive officers’ compensation, including preparing an analysis against the compensation of executive officers at comparable companies, to ensure that our compensation is market competitive, with the goal of retaining and adequately motivating our senior management. During 2021,2023, the Compensation Committee engaged Compensia to make recommendations for updating our compensation peer group, and to review and make recommendations regarding our equity plan and executive and director compensation for 2021.2023. Compensia was invited to attend Compensation Committee meetings where they presented and discussed their analysis and findings. For 2021,2023, with the assistance of Compensia, the Compensation Committee updated our compensation peer group, described below in the section entitled “Peer Companies and Market Compensation Data.”
In December
On an annual basis since 2018 December 2019, September 2020 and September 2021,most recently in March 2024, the Compensation Committee analyzed whether the work of Compensia as a compensation consultant has raised any conflict of interest, taking into consideration the following factors: (i) the provision of other services to ourthe Company by Compensia; (ii) the amount of fees from ourthe Company paid to Compensia as a percentage of the firm’s total revenue; (iii) Compensia’s policies and procedures that are designed to prevent conflicts of interest; (iv) any business or personal relationship of Compensia or the individual compensation advisors employed by the firm with an executive officer of ourthe Company; (v) any business or personal relationship of the individual compensation advisors with any member of the Compensation Committee; and (vi) any stock of ourthe Company owned by the individual compensation advisors employed by Compensia. The Compensation Committee determined, based on its analysis of the above factors, that the work of Compensia and the individual compensation advisors employed by Compensia has not raised any conflict of interest and the Compensation Committee is satisfied with the independence of Compensia.
Peer Group and Market Compensation Data
When making compensation decisions,
Each year the Compensation Committee, reviewswith the advice and analysis of its independent compensation consultant, Compensia, establishes a peer group as a reference point for assessing named executive officer target compensation against market competitive data.
The peer group of similarly-situated executive officers at companies that we consider to be our peers, taking into consideration the experience, position and functional role, level of responsibility and uniqueness of applicable skills of both our executive officers and those of our peers, and the demand and competitiveness for attracting and retaining an individual with each executive officer’s specific expertise and experience in the biotechnology industry. While this analysis is helpful in determining market-competitive compensation for senior management, leading to better attraction and retention of top-quality executive officers, it is only one factor in determining our executive officers’ compensation, andused by the Compensation Committee exercises its judgment in determiningmaking 2023 compensation decisions was comprised of the nature and extent of its use.following companies:
| | | | | | | | |
| Agios Pharmaceuticals (AGIO) | IGM Biosciences (IGMS) | Sana Biotechnology (SANA) |
| Beam Therapeutics (BEAM) | ImmunityBio (IBRX) | Sorrento Therapeutics (SRNEQ) |
| Celldex Therapeutics (CLDX) | Intellia Therapeutics (NTLA) | SpringWorks Therapeutics (SWTX) |
| CRISPR Therapeutics AG (CRSP) | Iovance Biotherapeutics (IOVA) | Turning Point Therapeutics (formerly TPTX) |
| Editas Medicine (EDIT) | Lyell Immunopharma (LYEL) | Ultragenyx Pharmaceutical (RARE) |
| Fate Therapeutics (FATE) | Mirati Therapeutics (formerly MRTX) | Xencor (XNCR) |
| Global Blood Therapeutics (formerly GBT) | Nektar Therapeutics (NKTR) | |
With the assistance of its compensation consultant, and after taking into consideration such factors as product development stage, market capitalization, acquisitions, number of employees and public status, the following companies were selected and approved byCompensia, the Compensation Committee considered several factors in September 2020 to comprise our compensation peer group:
| | | | | | | | |
Acceleron Pharma | Agios Pharmaceuticals | BeiGene |
bluebird bio | Blueprint Medicines | CRISPR Therapeutics |
Editas Medicine | Fate Therapeutics | Global Blood Therapeutics |
Immunomedics | Iovance Biotherapeutics | Mirati Therapeutics |
Moderna | MyoKardia | Nektar Therapeutics |
Sage Therapeutics | Sarepta Therapeutics | Ultragenyx Pharmaceutical |
The Compensation Committee reviews the compensation peer group periodically to reflect changes in market capitalization and other factors, including number of employees, acquisitions and product development stage, and revisesdetermining the companies to be included in the peer group accordingly. In this regard,for 2023 executive compensation decisions, including stage of development, market capitalization, number of employees, public status and length of time being public, primary location of operations and level of research and development expenditures and revenue. At the time the peer group was revisedselected, the market capitalization for the companies ranged from $600 million to $9.5 billion with headcounts ranging from 130 to 1120 employees, compared to Allogene’s 30-day average market capitalization of $2.383 billion and approved by theheadcount of 344 employees.
The Compensation Committee in September 2021intends to consist of:continue reviewing and revising the peer group annually to ensure that it continues to reflect publicly traded companies comparable to Allogene.
| | | | | | | | |
Acceleron Pharma | Agios Pharmaceuticals | Beam Therapeutics |
bluebird bio | CRISPR Therapeutics | Editas Medicine |
Fate Therapeutics | Global Blood Therapeutics | IGM Biosciences |
Instil Bio | Intellia Therapeutics | Iovance Biotherapeutics |
Lyell Immunopharma | Mirati Therapeutics | Nektar Therapeutics |
Rubius Therapeutics | Sana Biotechnology | Sorrento Therapeutics |
SpringWorks Therapeutics | Turning Point Therapeutics | Ultragenyx Pharmaceutical |
Xencor | | |
Executive Compensation Program and Compensation Decisions for the Named Executive Officers
The components of our
Our executive compensation program in 2021 were as follows:2023 primarily consisted of base salary, annual cash incentive plan, and long-term incentive program, each of which plays an important role in our pay for performance philosophy and in achieving our compensation program objectives. For each element of compensation, we target an overall executive compensation program that is competitive with market data.
2023 Annual Base SalarySalaries
The Compensation Committee believes base salaries should be consistent with the base salaries provided by companies in our peer group. In January 2023, the Compensation Committee performed its annual analysis of base salaries for our executive management team, including our Chief Executive Officer, using the peer group market data provided by Compensia. Based on this analysis, the Compensation Committee recommended no changes to Dr. Chang’s 2023 annual base salary, and the Board did not approve of any changes based on the Compensation Committee's recommendation. The base salaries for Mr. Moore and Dr. Roberts were determined in April and January 2023, respectively, upon their commencement of our executive officers are designedemployment with us, with reference to compensate them for day-to-day services rendered during the fiscal year. Appropriate base salaries are used to recognize the experience, skills, knowledge and responsibilities required of each executive officer and to allow us to attract and retain individuals capable of leading us to achieve our business goals in competitivepeer group market conditions.data provided by Compensia.
The base salaries of our executive officers are reviewed at least annually by the Compensation Committee and adjustments are made to reflect Company and individual performance, as well as competitive market practices. The Compensation Committee also takes into account subjective performance criteria, such as an executive officer’s ability to lead, organize and motivate others, develop the skills necessary to mature with us, set realistic goals to be achieved in their respective area, and recognize and pursue new business opportunities that enhance our growth and success. The Compensation Committee does not apply specific formulas to determine increases, but instead makes an evaluation of each executive officer’s contribution to our long-term success. Annual adjustments to base salaries are effective as of January 1 of each year, with mid-year adjustments to base salaries made under special circumstances, such as promotions, retention measures or an increase in responsibilities.
The 20212023 annual base salaries for our Named Executive Officers (including a comparison with the 2022 salary for our Chief Executive Officer) were as follows:
| | | | | | | | |
Name | | 2021
Base Salary
($) |
David Chang, M.D., Ph.D. | | 695,000 | |
Eric Schmidt, Ph.D. | | 475,000 | |
Rafael Amado, M.D. | | 600,000(1) |
Alison Moore, Ph.D. | | 475,000 | |
Veer Bhavnagri | | 464,000 | |
(1) Dr. Amado's salary was increased from $525,000 | | | | | | | | | | | | | | | | | | | | |
| | Base Salary | | Base Salary Change |
| Name and Position | | 2022 | | 2023 | |
David Chang, M.D., Ph.D. President and Chief Executive Officer | | $724,000 | | $724,000 | | 0% |
Timothy Moore Executive Vice President, Chief Technical Officer | | ― | | $525,000 | | ― |
Zachary Roberts, M.D., Ph.D. Executive Vice President, Research and Development and Chief Medical Officer | | ― | | $525,000 | | ― |
In response to $600,000our say on May 18, 2021 as described below.
On May 18, 2021,pay results at the 2023 annual meeting of stockholders, in January 2024, the Compensation Committee reviewed(and the performance and compensation ofBoard, with respect to Dr. Amado. To that date, Dr. Amado had several extraordinary achievements, including initiating our clinical trial of ALLO-316 in advanced or metastatic clear cell renal cell carcinoma, accelerating the advancement of our TurboCAR technology and achieving clearance of an IND for our first TurboCAR candidate, ALLO-605, in relapsed/refractory multiple myeloma, and advancing positive data from our clinical trials of ALLO-501 and ALLO-501A in relapsed/refractory non-Hodgkin lymphoma. In light of these achievements and as a retention measure, the Compensation Committee increased Dr. Amado’s annual base salary for the remainder of 2021 from $525,000 to $600,000.
After resolution of the clinical hold in January 2022, the Compensation CommitteeChang) reviewed the annual base salary and target annual cash bonus opportunityamounts of Dr. Chang, Dr. Amado, Dr. Schmidt, Dr.Mr. Moore and Dr. Roberts and determined that (i) the 2024 base salary amounts for Dr. Chang and Mr. Bhavnagri. In recognition of achievements and as a retention measure,Moore would remain unchanged (which was the Compensation Committee recommended our Board increasesecond consecutive year that Dr. Chang’s annual base salary for 2022 from $675,000 to $724,000,was not increased) and increase his target annual cash bonus opportunity from 60% of annual(ii) the base salary amount for Dr. Roberts would be increased by 1% to 65%bring his base compensation in line with approximately the 50th percentile of annual base salary for 2022. The Compensation Committee also approved the increase of Dr. Amado's annual base salary for 2022 from $600,000 to $615,000, Dr. Schmidt’s and Dr. Moore’s annual base salary for 2022 from $475,000 to $490,000, and Mr. Bhavnagri's annual base salary for 2022 from $464,000 to $480,000. In addition, the Compensation Committee increased Dr. Amado's, Dr. Schmidt's, Dr. Moore's and Mr. Bhavnagri's target annual cash bonus opportunity from 40% of annual base salary to 45% of annual base salary for 2022. On January 27, 2022, based on the Compensation Committee’s recommendation, our Board approved the increase to Dr. Chang’s annual base salary and target annual cash bonus opportunity.peer group.
2023 Annual Performance-Based Cash Bonus OpportunityIncentive
Our Named Executive Officers are eligible to receive annual performance-based cash bonuses,incentive, which are designed to provide appropriate incentives to our executive officers to achieve pre-established annual corporate goals and to reward them for individual performance towards these goals. The annual performance-based cash bonusincentive each current Named Executive Officer is eligible to receive is generally based on the extent to which we achieve the corporate goals that our Board establishes each year. At the end of the year, our Board and the Compensation Committee review our performance and approve the extent to which we achieved each of these annual corporate goals. Generally, our Board and the Compensation Committee, as applicable, will assess each Named Executive Officer’s individual contributions towards reaching our annual corporate goals but does not typically establish specific individual goals for our Named Executive Officers.
For 2021,2023, the target annual cash bonus opportunity of Dr. Chang was set at 60% of his annual base salaryincentive percentages were unchanged from 2022 and the targetwere as follows:
| | | | | | | | |
| Position | | 2023 Target Incentive % of
Base Salary |
| Chief Executive Officer* | | 65% |
| Other Named Executive Officers | | 45% |
*Target annual cash bonus opportunitiesincentive opportunity for each of Dr. Amado, Dr. Schmidt, Dr. Moore and Mr. Bhavnagri2023 for our Chief Executive Officer was set at 40% of each Named Executive Officer's annual base salary. unchanged from 2022.
Our Board and the Compensation Committee may award above-target bonuses, which was limited in 2021 to 130% of the target annual cash bonus opportunities,incentives for extraordinary performance.performance as measured against pre-established stretch goals.
The
Regarding the corporate goals used in our 20212023 annual performance-based cash bonus planincentive, we are not disclosing the specific targets or specific actual outcomes of these metrics. Because our corporate goals include highly sensitive competitive data, including clinical, regulatory, manufacturing and financial targets, as well as strategic collaboration objectives, we do not disclose these details because we believe that such disclosure would result in competitive harm to us. Revealing certain elements of these goals could potentially reveal insights about our clinical, regulatory, manufacturing and strategic plans or objectives that our competitors or potential collaborators could use against us. We purposely set these goals at challenging levels, and not every goal was fully achieved.
Our goals were proposed by management,centered around four overall strategic objectives, each of which contained more specific tactical and reviewedoperational core goals and approved by the Compensation Committee and our Board. Our Board considered and assigned a relative weight to each corporate goal to appropriately focus efforts on achievementscorresponding stretch goals that were intended to enhance stockholder value.more aggressive. The four categories in order of weighting were:
Our core corporate goals for 2021 and the relative weighting of each corporate goal were as follows:
•Deliver Phase 1 data and initiate the Phase 2 portion of the ALPHA2 trial of ALLO-501A by the end of 2021 (weighted at 40%); | | | | | | | | |
| Goal Category | Core Goal Weighting | Stretch Goal Weighting |
| Execute on Cema-cel Studies and Enhance CD19 Portfolio | 60% | 30% |
| Deliver on Product Manufacturing | 20% | 15% |
| Advance Pipeline | 10% | 5% |
| Manage Company Operations | 10% | 20% |
•Deliver data for a Phase 2 decision for ALLO-715 with or without nirogacestat by the end of 2021 (weighted at 20%);
•Obtain IND clearance for a Phase 1 clinical trial of ALLO-605 by the end of the second quarter of 2021 (weighted at 10%);
•Generate interim data from the Phase 1 clinical trial of ALLO-316 by the end of 2021 (weighted at 20%); and
•Produce a clinical GMP batch at our manufacturing facility in Newark, California by the end of 2021 (weighted at 10%).
Our Board also established additional, or stretch, corporate goals to complete by the end of 2021 as follows:
•Nominate a next-generation product candidate (weighted at 10%);
•Complete dosing of the first two cohorts in the Phase 1 clinical trial of ALLO-605 (weighted at 10%); and
•Complete the planned dose escalation of the Phase 1 clinical trial of ALLO-316 (weighted at 10%).
In December 2021, our Board reviewed our 2021 corporate goals and gave feedback to management and the Compensation Committee for further discussion and review in January 2022. Our Board and the Compensation Committee did not approve any annual bonus payment prior to the resolution of the clinical hold in early January 2022.
After the resolution of the clinical hold,2024, the Compensation Committee and our Board awarded partial (20%) credit tothoroughly reviewed the first corporate goal, as we reported interim clinical data from ALPHA2Company’s performance in achieving the 2023 goals and determined that our goals were achieved at the ASH annual meeting in December 2021, but did not initiate the Phase 2 trial. They also awarded partial (10%) credit to the second corporate goal as we finished dosing an initial cohort of patients to assess the ALLO-715 and nirogacestat combination, but we are following the patients prior to any decision on further enrollment or initiation of Phase 2. The Compensation Committee and our Board awarded full credit to the remaining corporate goals as all had been achieved. With respect to the stretch goals, the Compensation Committee and our Board awarded credit to the first stretch goal, but no credit to the other two stretch goals, which were not achieved.
In addition, the Compensation Committee and our Board awarded an additional 15% of credit for our perseverance in overcoming the clinical hold and certain additional accomplishments, including:
•Completed the first dose cohort in the ALLO-605 phase 1 study;
•Manufactured not just one, but three, clinical GMP batches at our manufacturing facility;
•Hired a Vice President, Commercial, and advanced an initial commercial strategy and roadmap;
•Executed on strategic business development and research initiatives; and
•Secured additional lab and office space at our headquarters to accommodate future company growth.
57.5%. As a result, the Compensation Committee approved awarding Dr. Schmidt, Dr. Amado, Dr. MooreRoberts and Mr. Bhavnagri 95%Moore 57.5% of their respective target annual cash bonus incentive
opportunity for 2021,2023, and recommended and our Board approved awarding Dr. Chang 95%57.5% of his target annual cash bonusincentive opportunity for 2021. In addition, due to Dr. Amado’s and Dr. Moore's exceptional scientific performance and leadership in overcoming the clinical hold in a timely and complete manner, the Compensation Committee awarded Dr. Amado a separate discretionary bonus of $100,000 and Dr. Moore a separate discretionary bonus of $75,000.2023. The 20212023 annual bonuscash incentive payments including Dr. Amado’s and Dr. Moore's discretionary bonuses, are summarized in the table below.
| | | | | | | | |
| Name | | | | | | | | 2023
Annual Cash Incentive
($) |
Name | | 2021
Annual Cash Bonus
($) |
| David Chang, M.D., Ph.D. | | 270,595 | | 396,150 |
Eric Schmidt, Ph.D.Timothy Moore (1) | | 93,416 | | 180,500 |
Rafael Amado,Zachary Roberts, M.D., Ph.D. (2) | | 134,727 | | 328,000 |
Alison Moore, Ph.D. | | 255,500 | |
Veer Bhavnagri | | 176,320 | |
(1) Pro-rated based on April 24, 2023 start date.
(2) Pro-rated based on January 3, 2023 start date.
2023 Long-Term Incentive Compensation
We provide long-term incentive compensation to our executive officers through the grant of equity awards. Our 2018 Equity Incentive Plan, as amended (the “EIP”), authorizes us to grant stock options, RSUs, PSUs and other equity-based awards. We believe that equity awards create incentives for our executive officers to further our long-term strategic plan to create long-term stockholder value. We also believe equity awards create an ownership culture. In addition, the vesting requirements of our equity awards contributescontribute to executive retention by providing an incentive to our executive officers to remain employed by us during the vesting period.
Generally, significant
For the 2023 equity awards, are granted at the time an executive officer commences employment. Thereafter, equity awards may be granted at varying times and in varying amounts in the discretion ofas explained above, the Compensation Committee or, if awards are being
grantedwas particularly concerned with retention. Being mindful of the need to our Chief Executive Officer,balance the Committee’s desire to structure compensation in a way that ensures pay-for-performance alignment and at the discretion of our Board, but are generally made once a year unless such executive officer is promoted, or for recognition of outstanding performance. None of our executive officers is currently party tosame time encourage retention and continuity within an employment agreement that provides for an automatic grant of stock options or other equity awards.
Historically, we have granted equity awards to our executive officers in the form of options to purchase shares of our common stock. In March 2019,organization, the Compensation Committee determined that an increasing number of companies in our compensation peer group grant full valuedecided to enhance the 2023 equity awards such as restricted stock unit ("RSU") awards and, therefore, decided that RSU awards should be addedwith PSUs having aggressive performance targets tied to our executive compensation program to attract and retain highly qualified executive officers. In this regard, while both stock options and RSU awards enable our executive officers to benefit, like stockholders, from any increases in the valueexecution of our commonclinical roadmap and stock stock options deliver future value only if the value of our common stock increases above the exercise price. In contrast, RSU awards deliver fully paid shares of our stock upon vesting, so, during periods of stock market volatility, RSU awards help retain employees. In addition, full value awards, such as RSU awards, are less dilutive to existing stockholders since fewer shares are needed to achieve an equivalent value relative to stock options.
The exercise price of our stock options is equal to the fair market value (our closing market price on the Nasdaq Global Select Market) of our common stock on the date of grant. Our stock options generally vest as to 1/4th of the shares subject to the option upon the first anniversary of the grant dateappreciation and 1/36th of the remaining shares each month thereafter until such award is fully vested on the four year anniversary of the grant date, subject to vesting acceleration as described under the heading “—Potential Payments Upon Termination or Change of Control” below. The RSU awards granted to our Named Executive Officers in 2021 generally vest in four equal annual installments from the grant date, subject to vesting acceleration as described under the heading “—Potential Payments Upon Termination or Change of Control” below. The other terms of the equity awards are governed by our 2018 Equity Incentive Plan, as amended (the “EIP”).
We have granted and may continue to grant RSU awards that vest upon performance conditions to select executive officers in orderdesigned to incentivize meeting specific strategic goals. the creation of long-term stockholder value.
In August 2019,March 2023, the Compensation Committee granted Dr. Amado a new hire performance RSU award for 57,361 shares of our common stock, which vests and settles in three equal installments, with (1) the first installment to vest upon initiation of a Phase 2 clinical trial of our first anti-CD19 product candidate, (2) the second installment to vest upon the filing of a biologics license application with the FDA for our first anti-CD19 product candidate, and (3) the third installment to vest upon FDA approval of our first anti-CD19 product candidate. Any unvested portion of the performance RSU award will expire after seven years.
The vesting schedule and the number of shares of our common stock granted are established to ensure a meaningful incentive to remain in our employ. Accordingly, the stock award will provide a return to the employee only if the employee remains in our service, and, in the case of the stock option component, only if the market price of our common stock appreciates over the option term. The Compensation Committee believes that the new hirerecommended an equity awards granted to Dr. Amado were necessary to secure his employment and to incentivize his long-term commitment, performance and support of our anti-CD19 product candidate, which is critical for our success.
In March 2021, the Compensation Committee granted our Named Executive Officers, and recommended a grant to our Chief Executive Officer that was approved by our Board,Board. This equity grant included stock options, to purchase shares of our common stockRSUs, and RSU awards that may be settled for shares of our common stock over a four year time-based vesting schedule described above.PSUs. In determining the number of shares of our common stock subject to the various equity awards, the Compensation Committee determined that the PSU component should comprise 50% of the total target dollar value of the award, 35% would be delivered as stock options, and RSU15% would be delivered as RSUs. The Compensation Committee determined the target dollar value based on competitive market data provided by Compensia. In determining the overall size and structure of the 2023 equity awards, granted to the Named Executive Officers, the Compensation Committee first determinednot only considered competitive market data provided by Compensia, but also considered that PSUs would comprise the valuelargest portion of overall annual equity awards, and then determined the appropriate award, mix. In March 2021,which the Compensation Committee choseintentionally tied to deliver a value mix of approximately 70% stock options and approximately 30% RSUs, based ontransformational performance outcomes. In recommending these equity awards, the accounting “fair value” as reflected in our Summary Compensation Table. The ratio of RSUs to stock options was based in part on an analysis of competitive market data prepared by the Compensation Committee’s compensation consultant and the Compensation Committee’s desire to tie a majority of the equity compensation program to stockholder value creation with stock options. The Compensation Committee also desired to provide a minority portion of the equity program with a durable retention profile, and the grant of RSU awards assists in retaining the team through periods of stock price volatility. The Compensation Committee also considered the equity awards granted at the 75th percentile to the executives holding comparable positions at the companies in our compensation peer group, as well as each Named Executive Officer’sof Dr. Chang’s existing equity holdings, level of responsibility and criticality, unvested status of existing equity holdings, and its subjective assessment of each Named Executive Officer’s individual performance and our overall companyCompany performance. As detailed below, achievement of the PSU goals will require successful execution of our clinical roadmap, culminating in FDA approval, and the creation of extraordinary stockholder value.
In addition, on May 18, 2021,
The target dollar value used by the Compensation Committee reviewedin establishing market-based PSU grants (i.e., PSUs wherein the vesting performance measure is based on achieving a target Allogene stock price) differs from the grant date fair value calculated under FASB ASC Topic 718 in which the value is discounted using the Monte Carlo simulation. For all other equity awards (i.e., stock options, RSUs, and performance-based PSUs) the target dollar value is the same as the grant date fair value calculated under FASB ASC Topic 718. The grant date fair values under FASB ASC Topic 718 are the values reflected in the compensation tables below.
The following table summarizes the CEO March 2023 equity grants:
| | | | | | | | | | | | | | |
| Type | | Number of Shares (#) | | Vesting |
| Stock options | | 1,492,200 | | | 1/4th on the first anniversary and 1/36th of the remaining shares each month thereafter |
| Restricted Stock Units | | 444,107 | | | 25% annually over four years |
| Performance Stock Units | | 1,480,357 | | | •50% of the award will vest, if at all, upon our stock price achieving a 30-day weighted average trading price of $18.00 per share (representing an increase of over 350% of the then current trading price) prior to January 3, 2025
•50% of the award will vest, if at all, upon the first FDA approval of a company product prior to January 3, 2028 |
| Equity Awards Aggregate Grant Date Fair Value | | $ | 13,067,253 | | | |
During 2023 Dr. Roberts and Mr. Moore were also given equity awards under our long-term incentive compensation program, which included stock options, RSUs and PSUs. Since they were hired in 2023, Dr. Roberts and Mr. Moore were given new hire equity awards which were sized taking into consideration the competitive market data provided by Compensia, as well as each of their experience and expected contributions.Additionally, because Dr. Amado. To that date,Roberts started in January 2023, he was also given a pro-rated annual PSU award as part of the March 2023 annual equity grants. In structuring Dr. Amado had several extraordinary achievements, including initiating our clinical trial of ALLO-316 in advanced or metastatic clear cell renal cell carcinoma, accelerating the advancement of our TurboCAR technologyRoberts’ and achieving clearance of an IND for our first TurboCAR candidate, ALLO-605, in relapsed/refractory multiple myeloma, and advancing positive data from our clinical trials of ALLO-501 and ALLO-501A in relapsed/refractory non-Hodgkin lymphoma. In light of these achievements and as a retention measure,Mr. Moore’s equity awards, the Compensation Committee granted an RSU awardfelt that may vest and be settled for 200,534 units,it was important to include PSUs with 33,422 vesting inperformance targets that would align their incentives with the PSU incentives of the other executive officers to ensure unified objectives among the executive officers.
one year, 66,844 vesting in two years and 100,268 vesting in three years. Each unit granted pursuant to the RSU award represents a contingent right to receive one share of our common stock for each unit that vests. The Compensation Committee viewed the equity award as a critical retention measure ahead of our plans for rapid advancement of our pipeline, including the potential initiation of a planned pivotal trial of ALLO-501A and potential future submission for approval of ALLO-501A.
The following table summarizes Dr. Roberts' and Mr. Moore's 2023 equity awards granted to our Named Executive Officers during 2021 were as follows:grants:
| | | | | | | | | | | | | | | | | | | | |
| Name | | Options to Purchase Shares of Our Common Stock (#) | | RSU Awards for Shares of Our Common Stock (#) | | Equity Awards (Aggregate Grant Date Fair Value) |
| David Chang, M.D., Ph.D. | | 298,836 | | 80,452 | | $9,070,304 |
| Eric Schmidt, Ph.D. | | 92,365 | | 24,866 | | $2,803,462 |
| Rafael Amado, M.D. | | 92,365 | | 225,400 | | $8,803,440 |
| Alison Moore, Ph.D. | | 92,365 | | 24,866 | | $2,803,462 |
| Veer Bhavnagri | | 82,469 | | 22,202 | | $2,503,104 |
| | | | | | | | | | | | | | | | | | | | |
| Type | | Number of Shares (#) | | Vesting |
| Timothy Moore | | Zachary Roberts | |
| Stock options | | 831,024 | | | 873,015 | | | 1/4th on the first anniversary and 1/36th of the remaining shares each month thereafter |
| Restricted Stock Units | | 186,219 | | | 251,908 | | | 25% annually over four years |
| Performance Stock Units | | 116,387 | | | 95,418 | | | •100% upon the filing of a biologics license application with the U.S. Food and Drug Administration (“FDA”) for ALLO-501A (now cema-cel) by the end of 2025, or •80% upon the filing of a biologics license application with the U.S. Food and Drug Administration (“FDA”) for cema-cel by the end of 2026, or •40% upon the filing of a biologics license application with the U.S. Food and Drug Administration (“FDA”) for cema-cel within 5 years of the grant |
| Performance Stock Units | | 116,386 | | | 95,418 | | | •100% upon FDA approval of cema-cel by the end of 2025, or •80% upon FDA approval of cema-cel by the end of 2026, or •40% upon FDA approval of cema-cel within 5 years of the grant |
| Performance Stock Units | | — | | | 148,035 | | | •33% of the award will vest, if at all, upon our stock price achieving a 30-day weighted average trading price of $18.00 per share prior to January 3, 2025 •67% of the award will vest, if at all, upon the first FDA approval of a company product prior to January 3, 2028 |
| Equity Awards Aggregate Grant Date Fair Value | | $ | 5,249,873 | | | $ | 7,384,155 | | | |
Health and Welfare Benefits
Our Named Executive Officers, during their employment with us, are eligible to participate in our employee benefit plans, including our medical, dental, group term life, disability and accidental death and dismemberment insurance plans, in each case on the same basis as all of our other employees. In addition, we provide a Section 401(k) plan to our employees, including our Named Executive Officers, as discussed in the section below entitled “—Section 401(k) Plan.”
Section 401(k) Plan
We maintain a defined contribution employee retirement savings plan (the “401(k) Plan”), for our employees. Our executive officers are eligible to participate in the 401(k) Plan on the same basis as our other employees. The 401(k) Plan is intended to qualify as a tax-qualified plan under Section 401(a) of the Code.Internal Revenue Code (the “Code”). The 401(k) Plan provides that each participant may contribute up to the lesser of 100% of compensation or the statutory limit, which was $19,500$22,500 for calendar year 2021.2023. Participants that are 50 years or older can also make “catch-up” contributions, which in calendar year 20212023 may have been up to an additional $6,500$7,500 above the statutory limit. We currently make matching contributions into the 401(k) Plan on behalf of participants. We match 100% of eligible contributions up to the first 3% of eligible compensation, with an additional match of 50% on the next 3% (maximum of 4.5%). Participant contributions are held and invested, pursuant to the participant’s instructions, by the plan’s trustee.
Perquisites and Other Personal Benefits
We generally do not provide perquisites or personal benefits to our Named Executive Officers. We do, however, pay a portion of the premiums for medical, dental, group term life, disability and accidental death and dismemberment insurance for all of our full-time employees. Our Board may elect to adopt qualified or nonqualified benefit plans in the future if it determines that doing so is in our best interests.
Agreements with our Named Executive Officers
We have entered into employment letter agreements with each of our Named Executive Officers. These letter agreements provide for at-will employment and set forth the Named Executive Officer’s initial base salary, eligibility for employee benefits and recommended equity grant. In 2020, we paid $83,256 to coveraddition, each of our Named Executive Officers has executed a form of our standard confidential information and invention assignment agreement. The key terms of the Hart-Scott-Rodino (“HSR”) filing fees forletter agreements with our Named Executive Officers are described below. Any potential payments and benefits due upon a qualifying termination of employment or a change in control of the Company are further described below under “—Potential Payments and Benefits upon Termination or Change in Control.”
David Chang, M.D., Ph.D. We entered into a letter agreement with Dr. Chang, which included a tax “gross-up” payment to coverour President and Chief Executive Officer, in June 2018 that governs the imputed income associatedcurrent terms of his employment with this payment. The filing and the associated filing fee were triggered by regulatory requirements becauseus. As described above, we have increased Dr. Chang's total holdingsannual base salary and annual target performance cash incentive over time, and his current base salary is $724,000 with an annual target performance cash incentive of 65% of his base salary.
Zachary Roberts, M.D., Ph.D. We entered into a letter agreement with Dr. Roberts, our Executive Vice President, Research and Development and Chief Medical Officer, in January 2023 that governs the current terms of his employment with us. Pursuant to the agreement, Dr. Roberts was entitled to an annual base salary of $525,000 and is eligible to receive an annual target performance cash incentive of 45% of his base salary. In 2024 we increased Dr. Robert’s annual base salary to $530,000. Dr. Roberts was paid a $75,000 sign-on advance that was considered earned when Dr. Roberts successfully completed one year of continuous employment with the Company. Dr. Roberts was granted an initial stock option to purchase 873,015 shares of our common stock. Dr. Roberts was also granted an initial RSU for 251,908 shares of our common stock, were abovewhich settle on an annual basis over four years. In addition, Dr. Roberts was awarded new hire PSUs for a specified value. Our Compensation Committeetotal of 190,836 shares, which will settle within a five-year period, if at all, based on the achievement of certain stated performance metrics.
Timothy Moore. We entered into a letter agreement with Mr. Moore, our Executive Vice President, Chief Technical Officer, in April 2023 that governs the current terms of his employment with us. Pursuant to the agreement, Mr. Moore was entitled to an annual base salary of $525,000 and Board determined it appropriateis eligible to payreceive an annual target performance cash incentive of 45% of his base salary. Mr. Moore was paid a $100,000 sign-on advance that was considered earned when Mr. Moore successfully completed one year of continuous employment with the HSR filing fees and associated taxes that arose solely asCompany. Mr. Moore was granted an initial stock option to purchase 831,024 shares of our common stock. Mr. Moore was also granted an initial RSU for 186,219 shares of our common stock, which settle on an annual basis over four years. In addition, Mr. Moore was awarded new hire PSUs for a resulttotal of 232,773 shares, which will settle within a five-year period, if at all, based on the achievement of certain stated performance metrics.
Each of the price appreciation in shares accumulated by Dr. Changoptions granted to our Named Executive Officers pursuant to their letter agreements are subject to a four-year vesting schedule, with 25% vesting one year after the vesting commencement date and the balance vesting monthly over a long period of time during which he had made substantial contributions toward such appreciation,the remaining 36 months, subject to each individual’s continued service through each vesting date.
For additional information regarding our long-term compensation and recognizing that our Boardequity grants, please see "Executive Compensation―Executive Compensation Program and Compensation Committee encourages him to hold our shares for alignment with stockholders’ interests. Our Board and Compensation Committee considers this reimbursement to be business relatedDecisions for the above reasons, even though the HSR filing fee is personally attributable to the individual executive. No HSR filing fees were dueNamed Executive Officers―Long-Term Incentive Compensation".
Potential Payments and Benefits Upon Termination or paidChange in 2021.Control
Post-Employment Compensation
Our executive officers, including our Named Executive Officers are entitled to certain severance and change of control payments and benefits pursuant to our change in control and severance benefit plan (the “Plan”), as described“Change in more detail below in the section entitled “—Potential Payments Upon Termination or Change of Control.” The Plan provides for a combination of a lump-sum cash severance payment, continued health benefits and acceleration of vesting on outstanding equity awards in specified circumstances. Acceleration of vesting is subject to a “double trigger” arrangement, meaning that vesting acceleration occurs only in the event of a change of control of the Company in connection with or followed by a termination of employment without cause by us, or with good reason by the Named Executive Officer.
Control Plan”). Given the industry in which we participate and the range of strategic initiatives that we may explore, we believe these arrangements are an essential element of our executive compensation program and assist us in recruiting and retaining talented individuals. In addition, since we believe it may be difficult for our executive officers to find comparable employment following an involuntary termination of employment in connection with or following a change of control of the Company, these payments and benefits are intended to ease the consequences to an executive officer of an unexpected termination of employment. By establishing these payments and benefits, we believe we can mitigate the distraction and loss of executive officers that may occur in connection with rumored or actual fundamental corporate changes and thereby protect stockholder interests while a transaction is under consideration or pending.
Accounting and Tax Considerations
Under Financial Accounting Standard Board ASC Topic 718, or ASC Topic 718, we are required to estimate and record an expenseThe Change in Control Plan provides for, each share-based payment award (including stock options) over the vesting period of the award. We record share-based compensation expense on an ongoing basis according to ASC Topic 718. The Compensation Committee has considered, and may in the future consider, the grantevent of performance-basedan involuntary termination of employment without “cause” or other types of stock awardsa resignation with “good reason,” and subject to our executive officers in lieureceipt of or in addition to stock optionsan effective waiver and RSU awards in lightrelease of the accounting impact of ASC Topic 718 and other considerations.
Under Section 162(m) of the Internal Revenue Code (“Section 162(m)”), compensation paid to any publicly held corporation’s “covered employees” that exceeds $1 million per taxable year for any covered employee is generally non-deductible. However, Section 162(m) provides a reliance period exception for corporations that became publicly held before December 20, 2019, pursuant to which the deduction limit under Section 162(m) does not apply to certain compensation paid (or in some cases, granted) pursuant to a plan or agreement that existed during the period in which the corporation was not publicly held, subject to certain requirements and limitations. Under Section 162(m), this reliance period ends upon the earliest of the following: (i) the expiration of the plan or agreement; (ii) the material modification of the plan or agreement; (iii) the issuance of all employer stock and other compensation that has been allocated under the plan; or (iv) the first meeting of stockholders at which directors are to be elected that occurs after the close of the third calendar year following the calendar year in which the corporation’s initial public offering occurs. However, the reliance period exception under Section 162(m) may be repealed or modified in the future as a result of certain changes that were made to Section 162(m) pursuant to the Tax Cuts and Jobs Act.
Compensation paid to each of the Company’s “covered employees” in excess of $1 million per taxable year generally will not be deductible unless it qualifies for the reliance period exception under Section 162(m). Because of certain ambiguities and uncertainties as to the application and interpretation of Section 162(m), as well as other factors beyond the control of the Compensation Committee, no assurance can be given that any compensation paid by the Company will qualify for the reliance period exception under Section 162(m) and be deductible by the Company in the future. Although the Compensation Committee will continue to consider tax implications as one factor in determining executive compensation, the Compensation Committee also looks at other factors in making its decisions and retains the flexibility to provide compensation for our Named Executive Officers in a manner consistent with the goals of our executive compensation program and the best interests of the Company and its stockholders, which may include providing for compensation that is not deductible by the Company due to the deduction limit under Section 162(m). The Compensation Committee also retains the flexibility to modify compensation that was initially intended to be exemptclaims from the deduction limit under Section 162(m) if it determines that such modifications are consistent with the Company’s business needs.
Risk Assessment Concerning Compensation Practices and Policies
The Compensation Committee annually reviews our compensation policies and practices to assess whether they encourage our employees to take inappropriate risks. After reviewing each of our compensation plans, and the checks and balances built into, and oversight of, each plan, in January 2022 the Compensation Committee determined that any risks arising from our compensation policies and practices for our employees are not reasonably likely to have a material adverse effect on our Company as a whole. In addition, the Compensation Committee believes that the mix and design of the elements of executive, compensation do not encourage management to assume excessive risks and, as described above under the heading “Compensation Discussion and Analysis,” significant compensation decisions, and decisions concerning the compensation of our executive officers, include subjective considerations by the Compensation Committee or our Board, which restrain the influence of formulae or objective factors on excessive risk taking. Finally, the mix of short-term compensation (in the form of base salary and annual cash bonus, if any), and long-term incentive compensation (in the form of stock options and RSU awards) also prevents undue focus on short-term results and helps align the interests of our executive officers with the interests of our stockholders.
Compensation Committee Interlocks and Insider Participation
As noted above, the Compensation Committee consists of David Bonderman, Franz B. Humer and John DeYoung. None of the members of the Compensation Committee during 2021 has at any time been our officer or employee. None of the members of the Compensation Committee during 2021 had a relationship that must be described under the SEC rules relating to disclosure of related
person transactions. None of our executive officers serve, or in the past fiscal year has served, as a member of the board of directors or the compensation committee of any entity that has one or more of its executive officers serving on our Board of Directors or Compensation Committee.
Compensation Committee Report*
The Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K with management and, based on such review and discussion, the Compensation Committee recommended to our Board of Directors that the Compensation Discussion and Analysis be included in this Proxy Statement and incorporated into the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021.
| | | | | | | | |
| | |
| | Compensation Committee |
| | David Bonderman, Chair
|
| | Franz B. Humer |
| | John DeYoung |
| | | | | |
* | The material in this report is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of the Company under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
2021 SUMMARY COMPENSATION TABLE
The following table sets forth all of the compensation awarded to, earned by or paid to our Named Executive Officers during the fiscal year ended December 31, 2021 and, with respect to Mr. Bhavnagri, during the fiscal year ended December 31, 2020, and, with respect to Dr. Chang, Dr. Schmidt, Dr. Amado and Dr. Moore, during the fiscal years ended December 31, 2020 and December 31, 2019.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and principal position | | Year | | Salary ($)(1) | | Bonus ($) | | Stock awards ($)(2) | | Option awards ($)(3) | | Non-equity incentive plan compensation ($)(4) | | All other compensation ($)(5) | | Total ($) |
| David Chang, M.D., Ph.D. | | 2021 | | 695,000 | | — | | 2,717,669 | | 6,352,635 | | 396,150 | | — | | 10,161,454 |
| President and Chief Executive Officer | | 2020 | | 675,000 | | — | | — | | 6,550,535 | | 506,250 | | 83,256 | | 7,815,041 |
| | 2019 | | 600,000 | | — | | 4,651,970 | | 5,327,140 | | 363,000 | | 250,000 | | 11,192,110 |
| Eric Schmidt, Ph.D. | | 2021 | | 475,000 | | | | 839,973 | | 1,963,489 | | 180,500 | | — | | 3,458,962 |
| Chief Financial Officer | | 2020 | | 440,000 | | — | | 561,176 | | 1,262,643 | | 220,000 | | — | | 2,483,819 |
| | 2019 | | 425,000 | | — | | 1,328,366 | | 1,519,700 | | 187,000 | | — | | 3,460,066 |
| Rafael Amado, M.D. | | 2021 | | 571,591 | | 100,000 | | 6,839,951 | | 1,963,489 | | 228,000 | | 11,125 | | 9,714,156 |
| Chief Medical Officer and Executive Vice President of Research and Development | | 2020 | | 510,000 | | 75,000 | | 561,176 | | 1,262,643 | | 255,000 | | — | | 2,663,819 |
| | 2019 | | 145,833 | | — | | 4,249,976 | | 2,759,623 | | 71,726 | | 225,000 | | 7,452,158 |
| Alison Moore, Ph.D. | | 2021 | | 475,000 | | 75,000 | | 839,973 | | 1,963,489 | | 180,500 | | 13,050 | | 3,547,012 |
| Chief Technical Officer | | 2020 | | 440,000 | | — | | 561,176 | | 1,262,643 | | 220,000 | | — | | 2,483,819 |
| | 2019 | | 425,000 | | — | | 996,275 | | 1,519,700 | | 187,000 | | — | | 3,127,975 |
| Veer Bhavnagri | | 2021 | | 464,000 | | | | 749,984 | | 1,753,120 | | 176,320 | | 13,050 | | 3,156,474 |
| General Counsel | | 2020 | | 425,000 | | — | | 537,490 | | 1,211,953 | | 212,500 | | 9,385 | | 2,396,328 |
(1) The dollar amount reported for 2019 in this column for Dr. Amado represents salary paid to Dr. Amado since the commencement of his employment (prorated based on an annual salary of $500,000).
(2) The dollar amounts reported for 2019 and 2020 in this column reflect the aggregate grant date fair value of restricted stock units granted during 2019 and 2020 based on the closing market price of the Company's common stock on the date of grant.
(3) The dollar amounts in this column represent the aggregate grant date fair valuea combination of stock option awards granted in 2019, 2020 and 2021. These amounts have been computed in accordance with FASB ASC Topic 718, using the Black-Scholes option pricing model. For a discussion of valuation assumptions, see Note 10 “Stock-based Compensation” to our financial statements included in our Annual Report on Form 10-K(1) cash severance for the year ended December 31, 2021. These amounts do not reflectseverance period and (2) the actual economic value that will be realized bypayment or reimbursement of premiums for continuation of coverage under group health plans for the Namedseverance period. The severance period is 24 months in the case of our Chief Executive Officer, uponand 12 months in the vesting of the stock options, the exercise of the stock options, or the sale of the common stock underlying such stock options.
(4) The dollar amounts in this column represent annual performance-based bonuses earned for 2019, 2020 and 2021. For more information, see above under “Annual Bonus Opportunity.”
(5) The dollar amounts in this column represent HSR filing fee and related tax gross-up to Dr. Chang in 2020, Section 401(k) Company contributions for Mr. Bhavnagri in 2021 and 2020, Section 401(k) Company contributions for Dr. Amado and Dr. Moore in 2021, relocation bonuses paid to Dr. Chang in 2019 and sign-on and relocation bonuses paid to Dr. Amado in 2019.
2021 GRANTS OF PLAN-BASED AWARDS TABLE
The following table sets forth information relating to the grant of plan-based incentive awards to our Named Executive Officers in 2021. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) | | All Other Stock Awards: Number of Shares of Stock or Units (#) | | All Other Option Awards: Number of Securities Underlying Options (#) | | Exercise or Base Price of Option Awards
($/Sh) | | Grant Date Fair Value of Stock and Option Awards
($) |
| Name | | Grant Date
| | Threshold
($) | | Target
($) | | Maximum
($) | | | | |
| David Chang, M.D., Ph.D. | | | | | | | | | | | | | | | | |
| Restricted stock unit award | | 3/26/2021 | | — | | | — | | | — | | | 80,452 | | | — | | | — | | | 2,717,669 | |
| Stock option award | | 3/26/2021 | | — | | | — | | | — | | | — | | | 298,836 | | | 33.78 | | | 6,352,635 | |
| Annual bonus | | — | | — | | | 396,150 | | | 594,225 | | | — | | | — | | | — | | | — | |
| Eric Schmidt, Ph.D. | | | | | | | | | | | | | | | | |
| Restricted stock unit award | | 3/26/2021 | | — | | | — | | | — | | | 24,866 | | | — | | | — | | | 839,973 | |
| Stock option award | | 3/26/2021 | | — | | | — | | | — | | | — | | | 92,365 | | | 33.78 | | | 1,963,489 | |
| Annual bonus | | — | | — | | | 180,500 | | | 270,750 | | | — | | | — | | | — | | | — | |
| Rafael Amado, M.D. | | | | | | | | | | | | | | | | |
| Restricted stock unit award | | 3/26/2021 | | — | | | — | | | — | | | 24,866 | | | — | | | — | | | 839,973 | |
| Restricted stock unit award | | 5/18/2021 | | — | | | — | | | — | | | 200,534 | | | — | | | — | | | 5,999,977 | |
| Stock option award | | 3/26/2021 | | — | | | — | | | — | | | — | | | 92,365 | | | 33.78 | | | 1,963,489 | |
| Annual bonus | | — | | — | | | 228,000 | | | 342,000 | | | — | | | — | | | — | | | — | |
| Alison Moore, Ph.D. | | | | | | | | | | | | | | | | |
| Restricted stock unit award | | 3/26/2021 | | — | | | — | | | — | | | 24,866 | | | — | | | — | | | 839,973 | |
| Stock option award | | 3/26/2021 | | — | | | — | | | — | | | — | | | 92,365 | | | 33.78 | | | 1,963,489 | |
| Annual bonus | | — | | — | | | 180,500 | | | 270,750 | | | — | | | — | | | — | | | — | |
| Veer Bhavnagri | | | | | | | | | | | | | | | | |
| Restricted stock unit award | | 3/26/2021 | | — | | | — | | | — | | | 22,202 | | | — | | | — | | | 749,984 | |
| Stock option award | | 3/26/2021 | | — | | | — | | | — | | | — | | | 82,469 | | | 33.78 | | | 1,753,120 | |
| Annual bonus | | — | | — | | | 176,320 | | | 264,480 | | | — | | | — | | | — | | | — | |
(1) All stock options and restricted stock unit awards were granted under the EIP.
NARRATIVE TO SUMMARY COMPENSATION TABLE AND GRANTS OF PLAN-BASED AWARDS TABLE
Agreements with our Named Executive Officers
We have entered into employment letter agreements with eachcase of our Named Executive Officers. These letter agreements provide for at-will employment and set forthother executive officers.
In the Named Executive Officer’s initial base salary, eligibility for employee benefits and recommended equity grant. In addition, each of our Named Executive Officers has executed a form of our standard confidential information and
invention assignment agreement. The key terms ofevent that the letter agreements with our Named Executive Officers are described below. Any potential payments and benefits due upon a qualifyinginvoluntary termination of employment oroccurs within the period commencing three months before and ending 12 months after a change in control of the Company, are further described below under “—Potential Payments and Benefits upon Termination orthen the participants in the Change in Control.”
David Chang, M.D., Ph.D. We entered into a letter agreement with Dr. Chang,Control Plan are entitled to the same benefits described above, but the severance period is 18 months for our President andexecutive officers other than our Chief Executive Officer. In addition, our Chief Executive Officer in June 2018 that governs the current termswould be entitled to 200% of his employment with us. Pursuant to the agreement, Dr. Chang wasannual target cash incentive and our other executive officers would be entitled to 150% of their annual target cash incentive, and each of our executive officers would be entitled to accelerated vesting of outstanding equity compensation awards.
Under the Change in Control Plan, the term “cause” generally means (i) the employee’s commission of any crime involving fraud, dishonesty or moral turpitude; (ii) the employee’s attempted commission of or participation in a fraud or act of dishonesty against us that results in (or might have reasonably resulted in) material harm to our business; (iii) the employee’s intentional, material violation of any contract or agreement between us and the employee or any statutory duty that the employee owes to us; or (iv) the employee’s conduct that constitutes gross insubordination, incompetence or habitual neglect of duties and that results in (or might have reasonably resulted in) material harm to our business. Under the Change in Control Plan, the term “change in control” generally means (1) the acquisition by any person or company of more than 50% of the combined voting power of our then outstanding stock, (2) a merger, consolidation or similar transaction in which our stockholders immediately before the transaction do not own, directly or indirectly, more than 50% of the combined voting power of the surviving entity (or the parent of the surviving entity) in substantially the same proportions as their ownership immediately prior to such transaction, (3) a sale, lease, exclusive license or other disposition of all or substantially all of our assets other than to an entity more than 50% of the combined voting power of which is owned by our stockholders in substantially the same proportions as their ownership of our outstanding voting securities immediately prior to such transaction, or (4) a complete dissolution or liquidation of the company.
Under the Change in Control Plan, the term “good reason” generally means (i) a material reduction of such employee’s annual base salary, which is a reduction of $525,000at least 10% of such employee’s base salary (unless pursuant to a salary reduction program applicable generally to the Company’s similarly situated employees); (ii) a material reduction in such employee’s authority, duties or responsibilities; (iii) a relocation of such employee’s principal place of employment with the Company (or successor to the Company, if applicable) to a place that increases such employee’s one-way commute by more than 50 miles as compared to such employee’s then-current principal place of employment immediately prior to such relocation (excluding regular travel in the ordinary course of business).
Stock Ownership Guidelines
We maintain stock ownership and was eligibleretention guidelines for our directors and executive officers to receiveensure that each of them has a long-term equity stake in Allogene, in order to both closely align the interests of directors and executive officers to those of our stockholders and to further our commitment to corporate governance.
Under the stock ownership guidelines, executive officers and directors must maintain stock ownership at a value equal to a multiple of their annual retainer or base salary, as applicable, as follows:
| | | | | |
| Position | Stock Retention Amount |
| Chief Executive Officer | 6 times executive’s base salary |
| Non-Employee Directors | 5 times director’s annual retainer |
| Other Executive Officers | 1 times executive’s base salary |
Under these guidelines, the compliance deadline for all of our executive officers and directors is the later of December 2023 and five years from an annual target performance bonusindividual's commencement of employment or appointment to our Board. Stock ownership that counts towards the requirement only includes shares of Common Stock and up to 45%. As described above, we50% of vested in-the-money stock options. Unvested stock options, unvested RSUs and PSUs are not included in determining compliance with these guidelines. The Compensation Committee annually monitors compliance with this policy. With the exception of one director, all Named Executive Officers and directors have increased Dr. Chang's annual base salarymet the stock ownership guidelines within the requisite time period. The one director who was not in compliance by December 31, 2023 has agreed with the Compensation Committee to a plan to reach compliance by the end of 2024.
Prohibition on Hedging, Pledging, and annual target performance bonus over time. In addition, Dr. Chang was paid a $250,000 relocation bonusSpeculative Trading
The Company considers it inappropriate for persons employed by or associated with the Company to engage in 2018 and was paid an additional relocation bonus of $250,000 in 2019 in connection with his relocationcertain transactions related to the San Francisco Bay Area. Dr. Chang was granted an initialsecurities of the Company (“Subject Securities”) that could result in their interests no longer being aligned with the same interests and objectives as other stockholders of the Company. Therefore, as part of our insider trading policy, Allogene prohibits our directors and employees from engaging in “hedging” or other inherently speculative transactions with respect to our common stock option to purchase 1,955,625 shares ofor borrowing against our common stock. Additionally, we entered into a vesting restriction agreementSpecifically, no officer, director, other employee or consultant may engage in short sales, transactions in put or call options, hedging transactions or other inherently speculative transactions with Dr. Chang in April 2018, pursuantrespect to which the 4,280,230 shares ofour common stock beneficially owned by Dr. Chang became subjectat any time. Certain hedging and monetization transactions involve the establishment of a short position in the Subject Securities and limit or eliminate a person’s ability to vesting overprofit from an increase in the value of the Subject Securities. Accordingly, these transactions can cause a 52-month period commencingperson’s interests to be misaligned with other stockholders of the Company. The Company therefore prohibits its directors, executive officers, and employees from engaging in December 2017, subject to his continuous service through each vesting date.such transactions.
Eric Schmidt, Ph.D. We entered into a letter agreement with Dr. Schmidt, our Chief Financial Officer, in June 2018 that governs the current terms of his employment with us. Pursuant to the agreement, Dr. Schmidt was entitled to an annual base salary of $375,000 and was eligible to receive an annual target performance bonus of up to 35%. As described above, we have increased Dr. Schmidt's annual base salary and annual target performance bonus over time. Dr. Schmidt was granted an initial stock option to purchase 1,464,750 shares of our common stock.
Rafael Amado, M.D. We entered into a letter agreement with Dr. Amado, our Chief Medical Officer and Executive Vice President of Research and Development, in July 2019 that governs the current terms of his employment with us. Pursuant to the agreement, Dr. Amado was entitled to an annual base salary of $500,000 and was eligible to receive an annual target performance bonus of up to 40% of his base salary. As described above, we have increased Dr. Amado's annual base salary and annual target performance bonus over time. In addition, Dr. Amado was paid a $75,000 sign-on advance and a $150,000 relocation bonus in 2019 in connection with his relocationno executive officer, director, other employee or consultant may margin, or make any offer to the San Francisco Bay Area. Dr. Amado was granted an initial stock option to purchase 160,630 shares of our common stock. Dr. Amado was also granted an initial restricted stock unit award for 105,162 sharesmargin, or otherwise pledge as security, any of our common stock, including without limitation, borrowing against such stock, at any time. Stock held in a margin account or pledged as collateral for a loan may be sold without a person’s consent if he or she fails to meet a margin call or defaults on a loan, which settle onmay occur at a time when the covered person is aware of material nonpublic information or is otherwise not permitted to trade in Company securities. Therefore, our directors, executive officers, employees and consultants are prohibited from engaging in these activities.
Clawback Policy
In November 2023, our Board adopted an annual basis over four years. In addition, Dr. Amado was awarded a new hire performance restricted stock unit award for 57,361 shares of our common stock, which settle incentive compensation recoupment policy (the “Clawback Policy”) in three equal installments,accordance with (1) the first installment to vest upon initiation of a Phase 2 clinical trial of our first anti-CD19 product candidate, (2) the second installment to vest upon the filing of a biologics license application with the FDA for our first anti-CD19 product candidate, and (3) the third installment to vest upon FDA approval of our first anti-CD19 product candidate. Any unvested portionRule 10D-1 of the performance restricted stock unit will expire after seven years.
Alison Moore, Ph.D. We entered into a letter agreement with Dr. Moore, our Chief Technical Officer, in May 2018Exchange Act and Nasdaq Rule 5608 that governsrequires the current terms of her employment with us. PursuantCompany to the agreement, Dr. Moorerecover excess incentive compensation that was entitledpaid to an annual base salary of $400,000 and was eligible to receive an annual target performance bonus of up to 35%. As described above, we have increased Dr. Moore's annual base salary and annual target performance bonus over time. In addition, Dr. Moore was paid a $100,000 relocation bonusexecutive officer based in 2018whole or in connection with her relocation to the San Francisco Bay Area. Dr. Moore was granted an initial stock option to purchase 892,500 shares of our common stock.
Veer Bhavnagri. We entered into a letter agreement with Mr. Bhavnagri, our General Counsel, in May 2018part on financial results that governs the current terms of his employment with us. Pursuant to the agreement, Mr. Bhavnagri was entitled to an annual base salary of $365,000 and was eligible to receive an annual target performance bonus of up to 35%. As described above, we have increased Mr. Bhavnagri's annual base salary and annual target performance bonus over time. In addition, Mr. Bhavnagri was paid a $50,000 sign-on bonus and a $75,000 relocation bonus in 2018 in connection with his relocation to the San Francisco Bay Area. Mr. Bhavnagri was granted an initial stock option to purchase 367,500 shares of our common stock. Additionally, we entered into a vesting restriction agreement with Mr. Bhavnagri in April 2018, pursuant to which the 262,500 shares of common stock beneficially owned by Mr. Bhavnagri became subject to vesting over a 52-month period commencing in December 2017, subject to his continuous service through each vesting date.
Each of the options granted to our Named Executive Officers arewere subject to a four-year vesting schedule, with 25% vesting one yearrestatement of the Company’s financial statements. The Clawback Policy specifies that following an accounting restatement, the Company must reasonably promptly recoup any compensation that is granted, earned or vested based wholly or in part upon the attainment of a financial reporting measure (“Incentive Compensation”) and which exceeds the amount that would have been received had such Incentive Compensation been determined based on the accounting restatement, unless the Compensation Committee determines that such recoupment would be impracticable. The Clawback Policy applies to all Incentive Compensation received during a three-year period preceding a restatement by a person (a) after beginning services as an executive officer, (b) who served as an executive officer at any time during the vesting commencement date and the balance vesting monthly over the remaining 36 months, subject to each individual’s continued service through each vesting date.
For additional information regarding our long-term compensation and equity grants, please see "Executive Compensation--Executive Compensation Program and Compensation Decisionsperformance period for the Named Executive Officers--Long-Termapplicable Incentive Compensation".Compensation and (c) while the Company has securities listed on Nasdaq or another national securities exchange or association.
CEO Pay Ratio
Our compensationIn February 2024, we restated previously issued financial statements for the years ended December 31, 2020, 2021 and benefits philosophy2022 and interim quarters during 2022 and 2023 due to non-cash accounting adjustments associated with the overall structure of our compensation and benefit programs are broadly similar across the organization. We strive to provide pay, benefits, and services that are competitive to market and create incentives to attract and retain employees. Our compensation package includes market-competitive pay, broad-based stock grants, health care and 401(k) plan benefits, paid time off and family leave, among others. We also provide annual incentive bonus opportunities that are tied to both company performance as well as individual performance to foster a pay-for-performance culture.
Under rules adopted pursuant to the Dodd-Frank Act, we are required to calculate and disclose the total compensation paid to our median paid employee, as well as the ratioformation of the total compensation paid to the median employee as compared to the total compensation paid to our Chief Executive Officer (the “CEO Pay Ratio”). The paragraphs that follow describe our methodology and the resulting CEO Pay Ratio.
Measurement and Methodology
We identified the median employee using our employee population onAllogene Overland Biopharm joint venture in December 31, 2021 (including all employees, whether employed on a full-time or part-time basis).
Under the relevant rules, we were required to identify the median employee by use2020. None of a “consistently applied compensation measure,” or CACM. We chose a CACM that closely approximates the total annual direct compensation of our employees. Specifically, we identified the median employee by looking at annual base pay, awarded annual cash incentive opportunity, and the grant date fair value for equity awards granted as of December 31, 2021 for all active employees as of that date. The value of our 401(k) plan and health and welfare benefits provided was excluded as all employees, including the Chief Executive Officer, are offered the same benefits. We did not perform adjustments to the compensation paid to part-time employeesexecutive officers on or after October 2, 2023 (the effective date of our Clawback Policy) was based on the attainment of a financial reporting measure, and therefore there was no incentive compensation that was paid to calculate what theyan executive officer which we would have been paid on a full-time basis.required to recoup under our Clawback Policy.
After applying our CACM methodology, we identified the median employee. Once the median employee was identified, we calculated the median employee’s annual target total direct compensation in accordance with the requirements of the Summary Compensation Table.
Pay Ratio
Our median employee compensation as calculated using Summary Compensation Table requirements was $307,290. Our Chief Executive Officer’s compensation as reported in the Summary Compensation Table was $10,161,454. Therefore, our CEO Pay Ratio is approximately 33:1.
This information is being provided for compliance purposes and is a reasonable estimate calculated in a manner consistent with SEC rules, based on our internal records and the methodology described above. The pay ratio reported by other companies may not be comparable to the pay ratio reported above, as other companies have different employee populations and compensation practices and may use different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios. Neither the Compensation Committee nor management of the Company used the CEO Pay Ratio measure in making compensation decisions.
2021 OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END TABLE2023 Outstanding Equity Awards at Fiscal Year-End Table
The following table presents information concerning equity awards held by our Named Executive Officers as of December 31, 2021. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Option Awards | | Stock Awards |
| Name | | Grant Date | | Number of Securities Underlying Unexercised Options (#)
Exercisable | | Number of Securities Underlying Unexercised Options (#)
Unexercisable | | Option Exercise Price
($) | | Option Expiration Date | | Number of Shares of Stock that have not Vested (#) | | Market Value of Shares of Stock that have not Vested ($) |
| David Chang, M.D., Ph.D.(1)(2)(5) | | 4/6/2018 | | — | | | — | | | — | | | — | | 246,938 | | | 3,684,315 | |
| 6/25/2018 | | — | | | — | | | 2.27 | | 6/25/2028 | | 162,969 | | | 2,431,497 | |
| 3/13/2019 | | 292,700 | | | — | | | 26.89 | | 3/13/2029 | | 86,500 | | | 1,290,580 | |
| 3/18/2020 | | 541,190 | | | — | | | 18.22 | | 3/18/2030 | | 12,544 | | | 187,156 | |
| 3/26/2021 | | 298,836 | | | — | | | 33.78 | | 3/26/2031 | | 80,452 | | | 1,200,344 | |
| | | | | | | | | | | | | |
| Eric Schmidt, Ph.D.(3)(6) | | 6/25/2018 | | — | | | — | | | 2.27 | | 6/25/2028 | | 183,094 | | | 2,731,762 | |
| 3/13/2019 | | 83,500 | | | — | | | 26.89 | | 3/13/2029 | | 24,700 | | | 368,524 | |
| 3/18/2020 | | 94,703 | | | — | | | 18.22 | | 3/18/2030 | | 35,644 | | | 531,808 | |
| 3/26/2021 | | 92,365 | | | — | | | 33.78 | | 3/26/2031 | | 24,866 | | | 371,001 | |
| | | | | | | | | | | | | |
| Rafael Amado, M.D.(4) | | 9/3/2019 | | 160,630 | | | — | | | 26.15 | | 9/3/2029 | | 109,942 | | | 1,640,335 | |
| 3/18/2020 | | 109,600 | | | — | | | 18.22 | | 3/18/2030 | | 23,100 | | | 344,652 | |
| 3/26/2021 | | 92,365 | | | — | | | 33.78 | | 3/26/2031 | | 24,866 | | | 371,001 | |
| 5/18/2021 | | — | | | — | | | — | | | — | | 200,534 | | | 2,991,967 | |
| | | | | | | | | | | | | |
| Alison Moore, Ph.D. | | 6/25/2018 | | 579,959 | | | — | | | 2.27 | | 6/25/2028 | | — | | | — | |
| 3/13/2019 | | 83,500 | | | — | | | 26.89 | | 3/13/2029 | | 18,525 | | | 276,393 | |
| 3/18/2020 | | 109,600 | | | — | | | 18.22 | | 3/18/2030 | | 23,100 | | | 344,652 | |
| 3/26/2021 | | 92,365 | | | — | | | 33.78 | | 3/26/2031 | | 24,866 | | | 371,001 | |
| | | | | | | | | | | | | |
| Veer Bhavnagri(1) | | 4/6/2018 | | — | | | — | | | — | | | — | | 15,144 | | | 225,952 | |
| 6/25/2018 | | 112,300 | | | — | | | 2.27 | | 6/25/2028 | | — | | | — | |
| 3/13/2019 | | 73,500 | | | — | | | 26.89 | | 3/13/2029 | | 21,700 | | | 323,764 | |
| 3/18/2020 | | 105,200 | | | — | | | 18.22 | | 3/18/2030 | | 22,125 | | | 330,105 | |
| 3/26/2021 | | 82,469 | | | — | | | 33.78 | | 3/26/2031 | | 22,202 | | | 331,254 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Option Awards | | Stock Awards |
| Name | | Grant Date | | Number of Securities Underlying Unexercised Options (#)
Exercisable (7) | | Number of Securities Underlying Unexercised Options (#)
Unexercisable | | Option Exercise Price
($) | | Option Expiration Date | | Number of Shares of Stock that have not Vested (RSUs) (#)
(2) | | Market Value of Shares of Stock that have not Vested (RSUs) ($) (8) | | Equity Incentive Plan Awards: Number of Unearned Shares or Units of Stock that have not
Vested (PSUs)
(#) | | Equity Incentive Plan Awards: Market Value of Unearned Shares or Units of Stock that have not Vested (PSUs) ($) (8) |
| David Chang, M.D., Ph.D. | | 3/13/2019 | | 292,700 | | | — | | | 26.89 | | 3/13/2029 | | — | | | | | — | | | — | |
| 3/18/2020 (1) | | 541,190 | | | — | | | 18.22 | | 3/18/2030 | | 5,488 | | | 17,616 | | | — | | | 17,616 | |
| 3/26/2021 | | 298,836 | | | — | | | 33.78 | | 3/26/2031 | | 40,266 | | | 129,125 | | | — | | | 129,125 | |
| 3/23/2022 | | 2,195,512 | | | — | | | 9.69 | | 3/23/2032 | | — | | | — | | | — | | | — | |
| 3/22/2023 | | 1,492,200 | | | — | | | 5.04 | | 3/22/2033 | | 444,107 | | | 1,425,583 | | | 1,480,357 (3) | | 4,751,946 | |
| | | | | | | | | | | | | | | | | |
| Timothy Moore | | 4/24/2023 | | 831,024 | | | — | | | 5.37 | | 4/24/2033 | | 186,219 | | | 597,763 | | | 93,110 (4) | | 298,883 | |
| | | | | | | | | | | | | | | | | |
| Zachary J. Roberts M.D., Ph.D. | | 1/20/2023 | | 873,015 | | | — | | | 6.55 | | 1/20/2033 | | 251,908 | | | 808,625 | | | 76,336 (5) | | 245,039 | |
| 3/22/2023 | | — | | | — | | | — | | | — | | | — | | | — | | | 148,035 (6) | | 475,192 | |
| | | | | | | | | | | | | | | | | |
(1) In connection with the issuance of the Company’s Series A and Series A-1 convertible preferred stock in April 2018, the Company’s founders, including Dr. Chang and Mr. Bhavnagri, agreed to modify their fully vested founders’ shares of common stock outstanding to include a forfeiture restriction that lapses based on their continued service to the Company. The number of shares of stock that have not vested is based on the founders’ shares that were beneficially owned by Dr. Chang and Mr. Bhavnagri as of December 31, 2021. The shares are subject to vesting over a 52-month period commencing in December 2017, subject to continuous service through each vesting date.
(2) On July 9, 2018, Dr. Chang elected to early exercise in full his stock option granted on June 25, 2018. We have a right to repurchase any unvested shares subject to such award if Dr. Chang ceases to provide services to us prior to the date on which all shares subject to the award have vested in accordance with the applicable vesting schedule. The June 25, 2018 option award is subject to a four-year vesting schedule, with 25% vesting one year after the vesting commencement date and the balance vesting monthly over the remaining 36 months. The vesting commencement date for such award was April 6, 2018.
(3) On June 26, 2018, Dr. Schmidt elected to early exercise in full his stock option granted on June 25, 2018. We have a right to repurchase any unvested shares subject to such award if Dr. Schmidt ceases to provide services to us prior to the date on which all shares subject to the award have vested in accordance with the applicable vesting schedule. The June 25, 2018 option award is subject to a four-year vesting schedule, with 25% vesting one year after the vesting commencement date and the balance vesting monthly over the remaining 36 months. The vesting commencement date for such award was June 18, 2018.
(4) A total of 57,361 of the stock awards granted on September 3, 2019 are performance based restricted stock units which vest upon achievement of specific performance criteria.
(5) On May 24, 2021, Dr. Chang elected to early exercise 14,897 of his stock option granted on March 18, 2020. We have a right to repurchase any unvested shares subject to such award if Dr. Chang ceases to provide services to us prior to the date on which all shares subject to the award have vested in accordance with the applicable vesting schedule. The March 18, 2020 option award is subject to a four-year vesting schedule, with 25% vesting one year after the vesting commencement date and the balance vesting monthly over the remaining 36 months. The vesting commencement date for such award was March 18, 2020.
(6) On May 24, 2021, Dr. Schmidt elected to early exercise 14,897 Unvested shares as of hisDecember 31, 2023 are presented as stock option granted on March 18, 2020. We have a right to repurchase any unvested shares subject to such award if Dr. Schmidt ceases to provide services to us prior to the date on which all shares subject to the award have vested in accordance with the applicable vesting schedule.awards. The March 18, 2020 option award is subject to a four-year vesting schedule, with 25% vesting one year after the vesting commencement dateMarch 13, 2021 and the balance vesting monthly over the remaining 36 months.
(2) The vesting commencementRSU awards vest in four equal annual installments from the grant date.
(3) 740,178 of PSU awards will vest, if at all, upon our stock price achieving a 30-day weighted average trading price of $18.00 per share prior to January 3, 2025, and 740,179 of PSU awards will vest, if at all, upon the first FDA approval of a Company product prior to January 3, 2028.
(4) 186,219 of PSU awards will vest, if at all, in two equal installments, with (a) the first installment to vest upon the filing of a biologics license application with the FDA for ALLO-501A, provided that 125% of such installment shall vest if such filing is completed by the end of 2025, 100% of such installment shall vest if such filing is completed in 2026 and 50% of such installment shall vest if such filing is completed after 2026, and (b) the second installment to vest upon FDA approval of ALLO-501A, provided that 125% of such installment shall vest if such approval is achieved by the end of 2025, 100% of such installment shall vest if such approval is achieved in 2026 and 50% of such installment shall vest if such approval is achieved after 2026. Any unvested portion of PSUs shall expire unvested after five years. Number of PSU awards in the table above is 93,110 and estimated based on the assumption that 50% of both installments will vest.
(5) 152,671 of PSU awards will vest, if at all, in two equal installments, with (a) the first installment to vest upon the filing of a biologics license application with the FDA for ALLO-501A, provided that 125% of such installment shall vest if such filing is completed by the end of 2025, 100% of such installment shall vest if such filing is completed in 2026 and 50% of such installment shall vest if such filing is completed after 2026, and (b) the second installment to vest upon FDA approval of ALLO-501A, provided that 125% of such installment shall vest if such approval is achieved by the end of 2025, 100% of such installment shall vest if such approval is achieved in 2026 and 50% of such installment shall vest if such approval is achieved after 2026. Any unvested portion of PSUs shall expire unvested after five years. Number of PSU awards in the table above is 76,336 and estimated based on the assumption that 50% of both installments will vest.
(6) 48,852 of PSU awards granted in 2023 will vest, if at all, upon our stock price achieving a 30-day weighted average trading price of $18.00 per share prior to January 3, 2025, and 99,183 of PSU awards will vest, if at all, upon the first FDA approval of a company product prior to January 3, 2028.
(7) Our stock options vest as to 1/4th of the shares subject to the option upon the first anniversary of the grant date forand 1/36th of the remaining shares each month thereafter until such award was March 18, 2020.is fully vested on the four year anniversary of the grant date.
2021 OPTION EXERCISES AND STOCK VESTED TABLE(8) Based on the closing market price of our common stock on December 29, 2023 of $3.21 per share.
Securities Authorized for Issuance under Equity Compensation Plans
The following table providessets forth the aggregate information of our equity compensation plans in effect as of December 31, 2023.
Equity Compensation Plan Information
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | Number of securities to be issued upon exercise of outstanding options, warrants and rights
(a) | | Weighted-average exercise price of outstanding options, warrants and rights
(b)($) | | Number of securities remaining available for issuance under equity compensation plans (excluding securities reflected in column (a))
(c) |
| Equity compensation plans approved by security holders | | — | | | — | | | — | |
| Amended and Restated 2018 Equity Incentive Plan (Prior Plan)(1) | | 1,358,596 | | | 5.42 | | | — | |
| Amended and Restated 2018 Equity Incentive Plan(2) | | 32,634,821 | | | 9.93 | | | 6,468,650 | |
| 2018 Employee Stock Purchase Plan(3) | | — | | | — | | | 6,469,951 | |
| Equity compensation plans not approved by security holders | | — | | | — | | | — | |
| Total | | 33,993,417 | | | 15.35 | | | 12,938,601 | |
(1) As of December 31, 2023, under our Amended and Restated 2018 Equity Incentive Plan (the “Prior Plan”), the number of outstanding awards under column (a) includes 1,358,596 shares which are issuable upon the exercise of outstanding options (including options that are immediately exercisable) at a weighted-average exercise price of $5.42.
(2) The EIP became effective on stock options exercised, includingSeptember 26, 2018, which replaced the Prior Plan. Initially, the aggregate number of shares of our common stock acquiredthat may be issued under the EIP is 20,432,250 shares. Additionally, on January 1 of each year, commencing on January 1, 2019 and ending on January 1, 2028, the number of shares authorized for issuance under the 2018 Plan is automatically increased by a number equal to: (a) 5% of the total number of shares of capital stock outstanding on December 31 of the preceding calendar year; or (b) such lesser number of shares of common stock as is determined by our Board or the Compensation Committee for the applicable year. All shares of our common stock reserved and available under the EIP shall constitute the maximum aggregate number of shares of common stock that may be issued through incentive stock options. As of December 31, 2023, the number of outstanding awards under column (a) includes: (1) 21,812,946 shares issuable upon the exercise of outstanding options at a weighted-average exercise price of approximately $9.93 and (2) 12,180,471 shares issuable upon the vesting of outstanding restricted stock units. The weighted-average exercise price shown in column (b) is for the outstanding options only. As of January 1, 2024, the number of shares issuable under the EIP increased to 62,528,644 shares, of which 14,900,761 shares remained available for issuance under the EIP.
(3) Our 2018 Employee Stock Purchase Plan (the “ESPP”) became effective on September 26, 2018. The ESPP authorizes the issuance of 1,160,000 shares of our common stock pursuant to purchase rights granted to our employees or to employees of any of our designated affiliates. On January 1 of each year, commencing on January 1, 2019 and ending on January 1, 2028, the number of shares authorized for issuance under the 2018 ESPP is automatically increased by a number equal to the lesser of: (a) 1% of the total number of shares of capital stock
outstanding on December 31 of the preceding calendar year; (b) 2,320,000 shares; or (c) such lesser number of shares of Common Stock as is determined by our Board or the Compensation Committee for the applicable year. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Code.
Pay Versus Performance
As required by Section 953(a) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 402(v) of Regulation S-K, we are providing the following information about the relationship between executive "compensation actually paid" and certain financial performance of our company. Use of the term "compensation actually paid" is required by the SEC's rules and as a result of the calculation methodology required by the SEC, such amounts differ from compensation actually received by the individuals and the value realized, determined ascompensation decisions described below, for ourin the "Discussion of Named Executive OfficersOfficer Compensation" section above.
| | | | | | | | | | | | | | | | | | | | |
| Pay Versus Performance |
| Year | Summary Compensation Table Total for Principal Executive Officer (“PEO”)(1) ($) | Compensation Actually Paid to PEO(2) ($) | Average Summary Compensation Table Total for Non-PEO Named Executive Officers (“NEOs”)(3) ($) | Average Compensation Actually Paid to Non-PEO NEOs(4) ($) | Value of Initial Fixed $100 Investment Based On: | Net Income (Loss) (thousands)(6) |
Total Shareholder Return (“TSR”)(5) ($) |
| (a) | (b) | (c) | (d) | (e) | (f) | (g) |
| 2023 | 14,061,848 | 1,289,577 | 6,967,886 | 2,717,460 | 12.36 | (327,265) |
| 2022 | 14,797,837 | 1,395,619 | 4,967,452 | (400,254) | 24.90 | (340,414) |
| 2021 | 10,161,454 | (2,306,242) | 4,969,151 | 642,739 | 57.63 | (182,051) |
(1) The dollar amounts reported in column (b) are the amounts of total compensation reported for Dr. Chang (our Chief Executive Officer) for each corresponding year in the “Total” column of the Summary Compensation Table. Refer to “2023 Summary Compensation Table.”
(2) The dollar amounts reported in column (c) represent the amount of “compensation actually paid” to Dr. Chang, as computed in accordance with Item 402(v) of Regulation S-K. The dollar amounts do not reflect the actual amount of compensation earned by or paid to Dr. Chang during the applicable year. In accordance with Item 402(v) of Regulation S-K, the following adjustments were made to Dr. Chang’s total compensation for each year ended December 31, 2021:to determine the compensation actually paid:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Option Awards | | Stock Awards |
| Name | | Number of Shares Acquired on Exercise (#) | | Value Realized on Exercise ($)(1) | | Number of Shares Acquired on Vesting (#) | | Value Realized on Vesting
($) |
| David Chang, M.D., Ph.D.(2) | | 27,410 | | | 190,774 | | | 987,756 | | | 25,389,434 | |
| Eric Schmidt, Ph.D. | | 14,897 | | | 103,683 | | | 386,238 | | | 10,216,429 | |
| Rafael Amado, M.D. | | — | | | — | | | 33,991 | | | 971,975 | |
| Alison Moore, Ph.D. | | — | | | — | | | 16,963 | | | 652,736 | |
| Veer Bhavnagri(2) | | 50,000 | | | 1,339,753 | | | 78,802 | | | 2,258,378 | |
| | | | | |
(1) | The value realized on exercise is based on the difference between the closing market price of our common stock on the date of exercise and the exercise price of the applicable options, and does not represent actual amounts received by the Named Executive Officers as a result of the option exercises. |
(2) | In connection with the issuance of the Company’s Series A and Series A-1 convertible preferred stock in April 2018, the Company’s founders, including Dr. Chang and Mr. Bhavnagri, agreed to modify their fully vested founders’ shares of common stock outstanding to include a forfeiture restriction that lapses based on their continued service to the Company. The shares are subject to vesting over a 52-month period commencing in December 2017, subject to continuous service through each vesting date. The shares vested in this table include a portion of the founders' shares that vested during 2021. |
| | | | | | | | | | | | | | | | | |
| Year | Reported Summary Compensation Table Total for PEO
($) | Reported Value of Equity Awards(a) ($) | Equity Award Adjustments(b) ($) | Compensation Actually Paid to PEO
($) |
| 2023 | 14,061,848 | (13,067,253) | 294,982 | 1,289,577 |
(a) The grant date fair value of equity awards represents the total of the amounts reported in the “Option Awards” and “Stock Awards” columns in the Summary Compensation Table for the applicable year.
(b) The equity award adjustments for each applicable year include the addition (or subtraction, as applicable) of the following: (i) the year-end fair value of any equity awards granted in the applicable year that are outstanding and unvested as of the end of the year; (ii) the amount of change as of the end of the applicable year (from the end of the prior fiscal year) in fair value of any awards granted in prior years that are outstanding and unvested as of the end of the applicable year; (iii) for awards that are granted and vest in same applicable year, the fair value as of the vesting date; (iv) for awards granted in prior years that vest in the applicable year, the amount equal to the change as of the vesting date (from the end of the prior fiscal year) in fair value; (v) for awards granted in prior years that are determined to fail to meet the applicable vesting conditions during the applicable year, a deduction for the amount equal to the fair value at the end of the prior fiscal year; and (vi) the dollar value of any dividends or other earnings paid on stock or option awards in the applicable year prior to the vesting date that are not otherwise reflected in the fair value of such award or included in any other component of total compensation for the applicable year. The valuations were performed using the Black-Sholes option pricing model, the lattice option pricing model or Monte Carlo
simulation. The valuation assumptions used to calculate fair values did not materially differ from those disclosed at the time of grant. The amounts deducted or added in calculating the equity award adjustments are as follows:
| | | | | | | | | | | | | | | | | |
| Year | Year End Fair Value of Outstanding and Unvested Equity Awards Granted in the Year
($) | Change in Fair Value of Outstanding and Unvested Equity Awards Granted in Prior Years as of the End of the Year
($) | Change in Fair Value of Equity Awards Granted in Prior Years that Vested in the Year
($) | Total Equity Award Adjustments
($) |
| 2023 | 4,878,379 | (1,761,911) | (2,821,486) | 294,982 |
(3) The dollar reported in column (d) represent the average of the amounts reported for our Company’s NEOs as a group (excluding Dr. Chang) in the “Total” column of the Summary Compensation Table in each applicable year. The names of each of the NEOs (excluding Dr. Chang) included for purposes of calculating the average amounts in each applicable year are as follows: for 2023, Dr. Roberts and Mr. Moore, and for 2022 and 2021, DIRECTOR COMPENSATION TABLEDr. Eric Schmidt, Dr. Rafael Amado, Dr. Alison Moore and Mr. Veer Bhavnagri, our former Chief Financial Officer, former Executive Vice President of Research and Development, former Chief Technical Officer, and former General Counsel, respectively.
(4) The dollar amounts reported in column (e) represent the average amount of “compensation actually paid” to the NEOs as a group (excluding Dr. Chang), as computed in accordance with Item 402(v) of Regulation S-K. The dollar amounts do not reflect the actual average amount of compensation earned by or paid to the NEOs as a group (excluding Dr. Chang) during 2023. In accordance with the requirements of Item 402(v) of Regulation S-K, the following adjustments were made to average total compensation for the NEOs as a group (excluding Dr. Chang) for 2023 to determine the compensation actually paid, using the same methodology described above in Note (2):
| | | | | | | | | | | | | | | | | |
| Year | Average Reported Summary Compensation Table Total for Non-PEO NEOs
($) | Average Reported Value of Equity Awards
($) | Average Equity Award Adjustments(a) ($) | Average Compensation Actually Paid to Non-PEO NEOs
($) |
| 2023 | 6,967,886 | (6,317,014) | 2,066,588 | 2,717,460 |
(a) The amounts deducted or added in calculating the total average equity award adjustments are as follows:
| | | | | | | | | | | |
| Year | Average Year End Fair Value of Outstanding and Unvested Equity Awards Granted in the Year
($) | Total Average Equity Award Adjustments
($) |
| 2023 | 2,066,588 | 2,066,588 |
(5) Cumulative TSR is calculated by dividing the sum of the cumulative amount of dividends for the measurement period, assuming dividend reinvestment, and the difference between our Company’s share price at the end and the beginning of the measurement period by our company’s share price at the beginning of the measurement period. No dividends were paid on stock or option awards in 2021, 2022 or 2023.
(6) The dollar amounts reported represent the amount of net income (loss) reflected in our consolidated audited financial statements for the applicable year.
Analysis of the Information Presented in the Pay Versus Performance Table
We generally seek to incentivize long-term performance, and therefore do not specifically align our performance measures with “compensation actually paid” (as computed in accordance with Item 402(v) of Regulation S-K) for a particular year. In accordance with Item 402(v) of Regulation S-K, we are providing the following descriptions of the relationships between information presented in the Pay Versus Performance table.
Compensation Actually Paid and Net Income (Loss)
Because we are a clinical-stage company, we did not have any product-based revenue during the periods presented. Consequently, we have not historically looked to net income (loss) as a performance measure for our executive compensation program. We also expect and have experienced higher net losses over time as we progress the development of our product candidates through clinical trials. Moreover, as a pre-commercial stage company with only limited, nonrecurring revenue associated with collaborations, we do not believe there is any meaningful relationship between our net loss and compensation actually paid to our NEOs during the periods presented.
Compensation Actually Paid and Cumulative TSR
The chart below shows the relationship between compensation actually paid to our PEO, the average of compensation actually paid to our non-PEO NEOs, and the Company's cumulative TSR over the three most recently completed fiscal years.
All information provided above under the “Pay Versus Performance” heading will not be deemed to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing, except to the extent the Company specifically incorporates such information by reference.
Risk Assessment Concerning Compensation Practices and Policies
The Compensation Committee is aware that compensation arrangements, if not properly designed, could encourage inappropriate or excessive risk taking. The Compensation Committee annually reviews our compensation policies and practices to assess whether they encourage our employees to take inappropriate risks. In the first quarter of 2024, our independent compensation consultant conducted a comprehensive independent risk assessment of our compensation policies and practices, and concluded that they do not create risks that are reasonably likely to have a material adverse effect on the Company. After reviewing the compensation consultant’s assessment, in March 2024 the Compensation Committee determined that any risks arising from our compensation policies and practices for our employees are not reasonably likely to have a material adverse effect on our Company as a whole. In addition, the Compensation Committee believes that the mix and design of the elements of executive compensation do not encourage management to assume excessive risks. Specifically, the mix of short-term compensation (in the form of base salary and annual cash incentive, if any), and long-term incentive compensation (in the form of stock options, RSU awards, and PSU awards) also prevents undue focus on short-term results and helps align the interests of our executive officers with the interests of our stockholders. Our stock options vest over multiple years and their value is not directly linked to the achievement of short term defined metrics. In addition, the Committee made the decision, starting in 2023, to award PSUs in addition to stock options and RSUs. The vesting of the PSUs are tied to the achievement of key milestones that are drivers of long-term stockholder value.
Our Board, upon the recommendation of the Compensation Committee, has implemented stock ownership guidelines, which ensure significant amounts of actual share ownership over time for our executive officers, mitigate excessive risk taking, and foster an ownership mentality among our senior leaders.
The Compensation Committee will continue to review risk as one of the elements it considers in the planning process for executive compensation in the future.
2023 Director Compensation Table
The following table shows for the fiscal year ended December 31, 20212023 certain information with respect to the compensation of all non-employee directors of the Company that served during 2021: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name | | Fees earned ($) | | Stock awards ($)(1) | | Option awards ($)(2) | | All other Compensation ($)(3) | | Total ($) |
| Elizabeth Barrett | | 18,750 | | | — | | 844,582 | | — | | | 863,332 |
| Arie Belldegrun, M.D. | | — | | | 1,811,790 | | 4,235,069 | | 726,910 | | | 6,773,769 |
| David Bonderman | | — | | | — | | — | | — | | | — |
| John DeYoung | | — | | | — | | — | | — | | | — |
| Franz Humer, Ph.D. | | 163,125 | | | 424,988 | | — | | — | | | 588,113 |
| Joshua Kazam | | 54,000 | | | 424,988 | | — | | 603,160 | | | 1,082,148 |
| Deborah Messemer | | 66,875 | | | — | | 424,988 | | — | | | 491,863 |
| Vicki Sato, Ph.D. | | 20,167 | | | 424,996 | | 422,284 | | — | | | 867,447 |
| Todd Sisitsky | | — | | | — | | — | | — | | | — |
| Owen Witte, M.D | | 50,000 | | | — | | 424,988 | | 27,233 | | | 502,221 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name | | Fees earned ($) | | Stock awards ($)(1) | | Option awards ($)(3) | | All other Compensation ($)(4) | | Total ($) |
| Elizabeth Barrett | | 52,500 | | | — | | 423,933 | | — | | | 476,433 |
| Arie Belldegrun, M.D. | | — | | | 5,331,258 (2) | | 3,380,244 | | 649,097 | | | 9,360,599 (5) |
| David Bonderman | | — | | | — | | — | | — | | | — |
| John DeYoung | | — | | | — | | — | | — | | | — |
| Franz Humer, Ph.D. | | 160,000 | | | 424,998 | | — | | — | | | 584,998 |
| Joshua Kazam | | 50,500 | | | — | | 423,993 | | 210,000 | | | 684,493 |
| Stephen Mayo, Ph.D. | | 40,000 | | | — | | 423,993 | | — | | | 463,993 |
| Deborah Messemer | | 70,000 | | | 424,998 | | — | | — | | | 494,998 |
| Vicki Sato, Ph.D. | | 54,000 | | | 339,999 | | 84,787 | | — | | | 478,786 |
| Todd Sisitsky | | — | | | — | | — | | — | | | — |
| Owen Witte, M.D | | 50,000 | | | 424,998 | | — | | 35,000 | | | 509,998 |
(1) The amounts reported in this column represent the aggregate grant date fair value of the restricted stock unit awardsRSUs granted during the year ended December 31, 2021,2023, as computed in accordance with Financial Accounting Standard Board Accounting Standards Codification Topic 718 for stock-based compensation transactions (ASC Topic 718) excluding the impact of estimated forfeitures related to service-based vesting conditions, which value is based on the closing market price of our common stock on the date of grant. As of December 31, 2021,2023, the aggregate number of restricted stock unitsRSUs outstanding held by our non-employee directors were: Dr. Belldegrun: 111,302;1,309,794; Dr. Humer: 16,498;37,281; Mr. Kazam: 16,498;zero; Dr. Mayo: 23,552; Ms. Messemer: 37,281; Dr. Sato: 36,279 and Dr. Sato: 19,362.Witte: 37,281. For a discussion of valuation assumptions, see Note 10 “Stock-based Compensation” to our financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2023.
(2) The dollar amounts of RSUs and PSUs granted to Dr. Belldergun reflect the aggregate grant date fair value of RSUs and PSUs computed in accordance with FASB ASC 718. The grant date fair value of the 2023 RSUs is based on the
closing market price of the Company's common stock on the date of grant. The grant date fair value of the PSUs is determined using Monte Carlo simulation for PSUs that vest upon achievement of a market condition. For the 2023 PSUs that vest upon achievement of a performance condition, the grant date fair value is based on the probable outcome of such condition. Assuming that maximum performance is achieved, the value of the PSUs with performance conditions that were granted to Dr. Belldegrun in 2023 would have been $2,487,003 at the date of grant under FASB ASC 718. 493,452 of PSU awards granted to Dr. Belldegrun with a grant date fair value of $1,352,058 will vest, if at all, upon our stock price achieving a 30-day weighted average trading price of $18.00 per share prior to January 3, 2025, and 493,453 of PSU awards with a grant date fair value of $2,487,003 will vest, if at all, upon the first FDA approval of a Company product prior to January 3, 2028. For a discussion of valuation assumptions, see Note 10 “Stock-based Compensation” to our financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2023.
(3) The dollar amounts in this column represent the aggregate grant date fair value of stock option awards granted in 2021.2023. These amounts have been computed in accordance with FASB ASC Topic 718, using the Black-Scholes option pricing model. For a discussion of valuation assumptions, see Note 10 “Stock-based Compensation” to our financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2021.2023. These amounts do not reflect the actual economic value that will be realized by the directors upon the vesting of the stock options, the exercise of the stock options, or the sale of the common stock underlying such stock options. As of December 31, 2021,2023, the aggregate number of shares outstanding under all option awards held by our non-employee directors were: Ms. Barrett: 63,385;250,575; Dr. Belldegrun: 394,356;2,847,489; Dr. Humer: 183,750; Mr. Kazam: 116,120; Dr. Mayo: 170,607; Ms. Messemer: 280,680;351,750; Dr. Sato: 34,692;93,451; and Dr. Witte: 54,040.89,575.
(3)(4) For Dr. Belldegrun, amount shown represents $463,000$482,600 in consulting fees paid to Bellco Capital LLC (“Bellco”) in 20212023 and an annual performance award of $263,910$166,497 paid to Bellco in 20222024 for services rendered in 2021.2023. Bellco is owned by Dr. Belldegrun, as co-trustee of the Belldegrun Family Trust, and Dr. Rebecka Belldegrun, as co-trustee of the Belldegrun Family Trust. For Mr. Kazam, amount shown represents consulting fees paid to Two River, LLC (“Two River”) in 2021.2023. Mr. Kazam provides consulting services to us through Two River and is a venture partner of Two River. For more information, see description of our consulting arrangement with Bellco and Two River below under “Transactions With Related Persons—Certain Related-Person Transactions—Consulting Arrangements”. For Dr. Witte, amount shown represents $27,233$35,000 in fees earned in connection with Dr. Witte's previous services on our scientific advisory board. In September 2023, Dr. Witte stepped down from the Company’s scientific advisory board and will no longer receive future compensation as a member.
(5) For a description of Dr. Belldegrun’s compensation, see the “―Executive Chair Compensation” section below.
We have reimbursed and will continue to reimburse all of our non-employee directors for their travel, lodging and other reasonable expenses incurred in attending meetings of our Board of Directors and committees of our Board of Directors, and will pay for the travel, lodging and other reasonable expenses incurred by our employee directors to attend meetings of our Board of Directors and, as applicable, committees of our Board of Directors.
Executive Chair Compensation
Dr. Belldegrun serves as our Executive Chair, and also provides consulting services through Bellco.his duties as such are described above under the heading “Information Regarding the Board of Directors and Corporate Governance—Board Leadership Structure.” Dr. Belldegrun contributes comprehensive, in-depth knowledgeaccepts no compensation under our Non-Employee Director Compensation Policy. Instead, in view of our business, cell therapy generally and the global life sciences industry, as well as valuable insights on leadership and strategy. Our Board believes that his advice and guidance to our senior leadership team and Board is essential to advancing our business.
Specifically,more expansive role, Dr. Belldegrun is actively involvedcompensated through the Company’s consulting agreement with Bellco. (See more information below under the heading “Transactions with Related Persons—Certain Related-Person Transactions—Consulting Arrangements.”)
The compensation paid to Bellco under the consulting agreement is reviewed annually by the Compensation Committee as both Executive Chairpart of the Committee’s annual executive compensation review process. Through this process, the Compensation Committee formulates a recommendation to the Board, and strategicthe Board is ultimately responsible for approving Dr. Belldegrun’s compensation under the Bellco consulting agreement. In consultation with its independent compensation consultant, including throughCompensia, the following activities:
•Presiding over all Board meetings;
•Attending weekly senior leadership team meetingsCompensation Committee formulates its recommendation for the Bellco compensation by selecting a compensation level that is a percentage of the CEO’s compensation. In determining the appropriate percentage of the CEO’s compensation to apply, the Compensation Committee considers data provided by its independent compensation consultant that analyzes executive chair compensation as a percentage of CEO compensation for life science companies deemed to be comparable to Allogene and off-site strategy meetings;which have an executive chair who has an
•Reviewingexpanded role similar to Dr. Belldegrun. The table below shows the results of this analysis done in December 2022 and approving Board agenda items;
•Helping to recruitDecember 2023, and assess potential directors;
•Overseeing Board and director evaluations;
•Reviewing Chief Executive Officer performance;
•Providing strategic advice to the Chief Executive Officer, as wellwhich served as the restbasis for setting Dr. Belldegrun’s compensation in 2023 and 2024, respectively.
Executive Chair Compensation as a Percentage of the senior leadership teamCEO Compensation
| | | | | | | | | | | | | | |
| Dec. 2022 50th Percentile
| Allogene
Chair 2023 Compensation | Dec. 2023 50th Percentile
| Projected
Allogene* Chair 2024 Compensation |
| Base Salary | 75% | 67% | 75% | 67% |
| Total Cash Comp. | 72% | 65% | 69% | 65% |
| Annual LTI Value | 72% | 67% | 72% | 67% |
| Total Compensation | 69% | 67% | 69% | 67% |
*Assumes CEO and our Board, includingExecutive Chair 2024 annual cash incentive is paid at 100% of target.
Based on operational, financial and strategic matters;
•Assisting with efforts to recruit and retain key executive talent;
•Assisting management in growing Allogene and advancing clinical trials;
•Communicating efforts with investors;
•Contributing key scientific and medical knowledge; and
•Assisting with key relationships, including collaborators, licensors and clinical trial sites.
Given Dr. Belldegrun's critical importance and deep involvement in Allogene,this analysis, the Compensation Committee recommendedformulated its recommendation for Dr. Belldegrun’s compensation under the Bellco consulting agreement to include the same three components included in our executive officer compensation program: (1) an annual cash retainer (paid in equal monthly installments) equal to about two-thirds of Allogene’s CEO base salary, (2) an annual cash incentive award targeted at 60% of Bellco’s annual cash retainer, and our(3) a long-term incentive award valued at about two-thirds of Allogene’s CEO annual long-term incentive award. Based on the Compensation Committee’s recommendations, these were the metrics used by the Board to set Dr. Belldegrun’s 2023 compensation, and in January 2024 the Board determined that no changes would be made for Dr. Belldegrun’s 2024 compensation. As a result, as illustrated in the table above, the Board concluded that Dr. Belldegrun’s 2023 and 2024 compensation would be set at slightly below the 50th percentile of the market data.
Consistent with the approach described above, and as reflected above in the 2023 Director Compensation Table, in consideration for the consulting services provided in 2023, we paid Bellco $482,600 in consulting fees in 2023. In addition, in January 2024, upon the recommendation of the Compensation Committee, the Board approved an equity granta 2023 annual cash incentive award to Bellco in the amount of $166,497, which was calculated as 57.5% (2023 corporate goal achievement level as determined by the Board) of Bellco’s target annual cash incentive award (i.e., 60% of $482,600). As further consideration of the consulting services, in March 20212023, upon the recommendation of the Compensation Committee, the Board awarded Bellco an annual long-term incentive award that included 994,800 stock options, 296,071 restricted stock units and 986,905 performance stock units, which was approximately two-thirds of Allogene’s CEO annual long-term incentive award.
The Company made no changes to the valueBellco compensation for 2024. We also reimburse Bellco for out of pocket expenses incurred in performing the grant to our CEO. consulting services.
Any compensation matters and decisions relating to Dr. Belldegrun and Bellco are reviewed outside of Dr. Belldegrun'sBelldegrun’s presence by both the Compensation Committee and the Board, and Dr. Belldegrun does not participate in the deliberations or determination of his own compensation.
Non-Employee Director Compensation Policy
Our 2023 non-employee director compensation policy includes:included:
•an annual cash retainer of $40,000;
•an additional annual cash retainer of $12,500, $7,500, $5,000 and $5,000$10,000 for service as a member of the Audit Committee, Compensation Committee, and the Nominating and Corporate Governance Committee, and Research and Development Committee, respectively;
•an additional annual cash retainer of $100,000, $25,000, $15,000, $10,000, and $10,000$20,000 for service as chair of the International and Business Development Oversight Committee, Audit Committee, Compensation Committee, and the Nominating and Corporate Governance Committee, and Research and Development Committee, respectively (in lieu of the committee member retainer above);
•an additional cash payment of $3,500 per meeting for each other member of the International and Business Development Oversight Committee; and $5,000 per annual Scientific Advisory Board meeting attended by a member of the Research and Development Committee;
•an initial option grant, vesting in 36 equal monthly installments, and/or an initial restricted stock unit award, vesting annually (or, commencing in 2023, semi-annually in the case of restricted stock unit awards) over a three-year period from the date of grant, having an aggregate grant date value of $850,000, with the director designating the proportionate share between the initial option grant and initial restricted stock unit award prior to or on the date of grant; and
•an annual option grant, vesting in 12 equal monthly installments, and/or an annual restricted stock unit award, vesting on the one-year anniversary of the date of grant (or, commencing in 2023, semi-annually over a one-year period in the case of restricted stock unit awards), having an aggregate grant date value of $425,000, with the director designating the proportionate share between the annual option grant and annual restricted stock unit award prior to or on the date of grant. The annual grants will be made on the date of each of our annual meetings of stockholders.
Each of the equity awards described above will vest and become exercisable subject to the non-employee director’s continuous service with us through each applicable vesting date, provided that each option and restricted stock unit award will vest in full upon a change of control of the Company, as defined under our EIP. The stock options and restricted stock unit awards will be granted under our EIP.
In consultation with Compensia, the Compensation Committee and specifically Mr. Bonderman and Mr. DeYoung reviewed the non-employee director compensation policy and concluded that no changes were warranted in 2021.2023. Neither Mr. Bonderman nor Mr. DeYoung accept any compensation for serving on our Board of Directors. In March 2024, however, upon the recommendation of the Compensation Committee, our Board approved an amendment to our non-employee director compensation policy which lowers the grant date fair value of any initial option grants for new non-employee directors from $850,000 to $600,000 and lowers the grant date fair value of the annual option grants from $425,000 to $300,000. The Compensation Committee recommended these changes following a review of non-employee director compensation peer data with Compensia. The new compensation levels were set at the 50th percentile of the peer data, which decreased from the prior year’s assessment.
SECURITIES AUTHORIZED FOR ISSUANCE UNDER EQUITY COMPENSATION PLANS
| | | | | | | | |
Security Ownership or Certain Beneficial Owners and Management
|
The following table sets forth certain information regarding the aggregate informationownership of our equity compensation plans in effectthe Company’s common stock as of DecemberMarch 31, 2021.
Equity Compensation Plan Information
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | Number of securities to be issued upon exercise of outstanding options, warrants and rights
(a) | | Weighted-average exercise price of outstanding options, warrants and rights
(b)($) | | Number of securities remaining available for issuance under equity compensation plans (excluding securities reflected in column (a))
(c) |
| Equity compensation plans approved by security holders | | — | | | — | | | — | |
| Amended and Restated 2018 Equity Incentive Plan (Prior Plan)(1) | | 2,454,684 | | | 4.78 | | | — | |
| Amended and Restated 2018 Equity Incentive Plan(2) | | 12,045,591 | | | 21.10 | | | 15,801,927 | |
| 2018 Employee Stock Purchase Plan(3) | | — | | | — | | | 4,551,912 | |
| Equity compensation plans not approved by security holders | | — | | | — | | | — | |
| Total | | 14,500,275 | | | 25.88 | | | 20,353,839 | |
(1) As2024 by: (i) each director; (ii) each of December 31, 2021, under our Amendedthe Company’s named executive officers; (iii) all current executive officers and Restated 2018 Equity Incentive Plan (the “Prior Plan”),directors of the numberCompany as a group; and (iv) all those known by the Company to be beneficial owners of outstanding awards under column (a) includes 2,454,684 shares which are issuable upon the exercise of outstanding options (including options that are immediately exercisable) at a weighted-average exercise price of $4.78.
(2) The EIP became effective on September 26, 2018, which replaced the Prior Plan. Initially, the aggregate number of shares of our common stock that may be issued under the EIP is 20,432,250 shares. Additionally, on January 1 of each year, commencing on January 1, 2019 and ending on January 1, 2028, the number of shares authorized for issuance under the 2018 Plan is automatically increased by a number equal to: (a)more than 5% of its common stock.
The table is based upon information supplied by officers, directors and principal stockholders, Schedules 13D and 13G filed with the total numberSEC and other sources believed to be reliable by the Company. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, the Company believes that each of the stockholders named in this table has sole voting and investment power with respect to the shares of capital stockindicated as beneficially owned. Applicable percentages are based on 170,452,967 shares outstanding on DecemberMarch 31, of2024, adjusted as required by rules promulgated by the preceding calendar year; or (b) such lesserSEC. The number of shares of common stock as is determined by our Board orused to calculate the Compensation Committee forpercentage ownership of each listed beneficial owner includes the applicable year. All shares of our common stock reserved and available under the EIP shall constitute the maximum aggregate number of shares of common stock underlying options or convertible securities held by such beneficial owner that may be issued through incentive stock options. Asare exercisable or convertible within 60 days following March 31, 2024. Unless otherwise indicated, the address for each person or entity listed in the table is c/o Allogene Therapeutics, Inc., 210 East Grand Avenue, South San Francisco, California 94080.
| | | | | | | | | | | | | | |
| Name of Beneficial Owner | | Number of shares Beneficially Owned | | Percentage Beneficially Owned |
| Greater than 5% Stockholders | | | | |
| Fidelity Management Research LLC(1) | | 25,241,499 | | 14.8% |
| Pfizer Inc.(2) | | 22,032,040 | | 12.9% |
| TPG GP A, LLC(3) | | 18,716,306 | | 11.0% |
| BlackRock, Inc.(4) | | 10,001,804 | | 5.9% |
| Vanguard Group(5) | | 9,030,825 | | 5.3% |
| Directors and Named Executive Officers | | | | |
| David Bonderman(6) | | 18,716,306 | | 11.0% |
| Arie Belldegrun, M.D.(7) | | 9,983,329 | | 5.8% |
| David Chang, M.D., Ph.D.(8) | | 10,047,561 | | 5.7% |
| Joshua Kazam(9) | | 419,183 | | * |
| Franz Humer, Ph.D.(10) | | 412,922 | | * |
| Deborah Messemer(11) | | 376,925 | | * |
| Owen Witte, M.D.(12) | | 345,126 | | * |
| Elizabeth Barrett(13) | | 250,575 | | * |
| Vicki Sato, Ph.D.(14) | | 155,272 | | * |
| Todd Sisitsky | | — | | — |
| John DeYoung | | — | | — |
| Stephen Mayo(15) | | 172,383 | | * |
| Zachary J Roberts, M.D.(16) | | 911,302 | | * |
| Earl Douglas(17) | | 960,854 | | * |
| Timothy Moore(18) | | 831,024 | | * |
| Geoffrey Parker(19) | | 950,190 | | * |
| All current executive officers and directors as a group (16 persons)(20) | | 44,532,952 | | 24.3% |
* Represents beneficial ownership of December 31, 2021, the numberless than 1%.
(1) Consists of outstanding awards under column (a) includes: (1) 10,239,167 shares issuable upon the exercise of outstanding options at a weighted-average exercise price of approximately $21.10 and (2) 4,261,108 shares issuable upon the vesting of outstanding restricted stock units. The weighted-average exercise price shown in column (b) is for the outstanding options only. As of January 1, 2022, the number of shares issuable under the EIP increased to 46,874,61825,241,499 shares of which 22,933,080 shares remained available for issuance under the EIP.
(3) Our 2018 Employee Stock Purchase Plan (the “ESPP”) became effective on September 26, 2018. The ESPP authorizes the issuance of 1,160,000 shares of our common stock pursuant to purchase rights granted to our employees or to employees of any of our designated affiliates. On January 1 of each year, commencing on January 1, 2019 and ending on January 1, 2028, the number of shares authorized for issuance under the 2018 ESPP is automatically increasedheld by a number equal to the lesser of: (a) 1% of the total number of shares of capital stock outstanding on December 31 of the preceding calendar year; (b) 2,320,000 shares; or (c) such lesser number of shares of Common Stock as is determined by our Board or the Compensation Committee for the applicable year. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Code.
POTENTIAL PAYMENTS UPON TERMINATION OR CHANGE IN CONTROL
Our executive officers, including our Named Executive Officers, are entitled to certain severance and change of control payments and benefits pursuant to our change in control and severance benefit plan (the “Change in Control Plan”Fidelity Management & Research Company LLC (“FMR LLC”). The Change in Control Plan provides for, inaddress of FMR LLC is 245 Summer Street, Boston, Massachusetts 02210. This information is based on the event of an involuntary termination of employment without “cause” or a resignationSchedule 13G filed on February 9, 2024 with “good reason,” and subject to our receipt of an effective waiver and release of claims from the executive, a combination of (1) cash severance for the severance period and (2) the payment or reimbursement of premiums for continuation of coverage under group health plans for the severance period. The severance period is 24 months in the case of our Chief Executive Officer, and 12 months in the case of our other Named Executive Officers.
In the event that the involuntary termination of employment occurs within the period commencing three months before and ending 12 months after a change in control of the Company, then the participants in the Change in Control Plan are entitled to the same benefits described above, but the severance period is 18 months for our Chief Financial Officer, Chief Medical Officer and Executive Vice President of Research and Development, Chief Technical Officer and General Counsel. In addition, our Chief Executive Officer would be entitled to 200% of his annual target bonus and our other Named Executive Officers would be entitled to 150% of each Named Executive Officer's annual target bonus, and each of our Named Executive Officers would be entitled to accelerated vesting of outstanding equity compensation awards.
Under the Change in Control Plan, the term “cause” generally means (i) the employee’s commission of any crime involving fraud, dishonesty or moral turpitude; (ii) the employee’s attempted commission of or participation in a fraud or act of dishonesty against usSEC.
that results in (or might have reasonably resulted in) material harm to our business; (iii)(2) Consists of 22,032,040 shares of common stock held by Pfizer. The address of Pfizer is 235 E. 42nd Street, New York, NY 10017. This information is based on the employee’s intentional, material violationSchedule 13D/A filed on October 25, 2023 with the SEC.
(3) Consists of any contract or agreement between us andan aggregate of 18,716,306 shares of common stock. TPG GP A is the employee or any statutory duty thatmanaging member of TPG Group Holdings (SBS) Advisors, LLC, a Delaware limited liability company, which is the employee owes to us; or (iv) the employee’s conduct that constitutes gross insubordination, incompetence or habitual neglectgeneral partner of duties and that results in (or might have reasonably resulted in) material harm to our business. The term “change in control” generally means (1) the acquisition by any person or company of more than 50%TPG Group Holdings (SBS), L.P., a Delaware limited partnership, which holds 100% of the combined voting powershares of our then outstandingClass B common stock (2)(which represents a merger, consolidation or similar transaction in which our stockholders immediately before the transaction do not own, directly or indirectly, more than 50%majority of the combined voting power of the surviving entity (orcommon stock) of TPG Inc., a Delaware corporation (“TPG”), which is the parentcontrolling shareholder of TPG GP Co, Inc., a Delaware corporation, which is the surviving entity) in substantiallymanaging member of TPG Holdings I-A, LLC, a Delaware limited liability company, which is the same proportions as their ownership immediately priorgeneral partner of TPG Operating Group I, L.P., a Delaware limited partnership, which is the sole member of each of (i) TPG GenPar VII Advisors, LLC, a Delaware limited liability company and (ii) The Rise Fund GenPar Advisors, LLC, a Delaware limited liability company. TPG GenPar VII Advisors, LLC is the general partner of TPG GenPar VII, L.P., a Delaware limited partnership, which is the general partner of TPG Carthage Holdings, L.P., a Delaware limited partnership, which directly holds 12,477,536 shares of Common Stock. The Rise Fund GenPar Advisors, LLC is the general partner of The Rise Fund GenPar, L.P., a Delaware limited partnership, which it the general partner of The Rise Fund Carthage, L.P., a Delaware limited partnership (together with TPG Carthage Holdings, L.P., the “TPG Funds”), which directly holds 6,238,770 shares of Common Stock. Because of TPG GP A’s relationship with the TPG Funds, TPG GP A may be deemed to such transaction, (3) a sale, lease, exclusive license or other dispositionbeneficially own the shares of all or substantially all of our assets other than to an entity more than 50% ofCommon Stock held by the combined voting power of whichTPG Funds. TPG GP A is owned by our stockholders in substantiallyentities owned by Messrs. Bonderman, Coulter and Winkelried. Because of the same proportions as theirrelationship of Messrs. Bonderman, Coulter and Winkelried to TPG GP A, each of Messrs. Bonderman, Coulter and Winkelried may be deemed to beneficially own the shares of Common Stock held by the TPG Funds. Messrs. Bonderman, Coulter and Winkelried disclaim beneficial ownership of our outstanding voting securities immediately priorthe shares of Common Stock held by the TPG Funds except to such transaction, or (4) a complete dissolution or liquidationthe extent of their pecuniary interest therein. The address of each of the company.
The term “good reason” generally means (i) a material reduction of such employee’s annual base salary, whichTPG Funds is a reduction of at least 10% of such employee’s base salary (unless pursuant to a salary reduction program applicable generally to the Company’s similarly situated employees); (ii) a material reduction in such employee’s authority, duties or responsibilities; (iii) a relocation of such employee’s principal place of employment with the Company (or successor to the Company, if applicable) to a place that increases such employee’s one-way commute by more than 50 miles as compared to such employee’s then-current principal place of employment immediately prior to such relocation (excluding regular travel in the ordinary course of business).
The following table provides an estimate of the potential payments and benefits pursuant to the Change in Control Plan, which could occur upon termination of the employment of our Named Executive Officers, including in connection with a change of control of the
Company, assuming a triggering event occurred on December 31, 2021: | | | | | | | | | | | | | | | | | | | | |
| Name | | Benefit | | Termination without Cause or Resignation for Good Reason Not in Connection with a Change in Control
($) | | Termination without Cause or Resignation for Good Reason in Connection with a Change in Control
($) |
| David Chang, M.D., Ph.D. | | Lump Sum Cash Severance Payment | | 1,390,000 | | | 1,390,000 | |
| | Lump Sum Target Bonus Payment | | — | | | 834,000 | |
| | Health Insurance Premiums | | 35,886 | | | 35,886 | |
| | Vesting Acceleration(1) | | — | | | 4,552,482 | |
| | Benefit Total | | 1,425,886 | | | 6,812,368 | |
| Eric Schmidt, Ph.D. | | Lump Sum Cash Severance Payment | | 475,000 | | | 712,500 | |
| | Lump Sum Target Bonus Payment | | — | | | 285,000 | |
| | Health Insurance Premiums | | 29,787 | | | 44,680 | |
| | Vesting Acceleration(1) | | — | | | 3,400,316 | |
| | Benefit Total | | 504,787 | | | 4,442,496 | |
| Rafael Amado, M.D. | | Lump Sum Cash Severance Payment | | 600,000 | | | 900,000 | |
| | Lump Sum Target Bonus Payment | | — | | | 360,000 | |
| | Health Insurance Premiums | | 18,322 | | | 27,483 | |
| | Vesting Acceleration(1) | | — | | | 5,347,955 | |
| | Benefit Total | | 618,322 | | | 6,635,438 | |
| Alison Moore, Ph.D. | | Lump Sum Cash Severance Payment | | 475,000 | | | 712,500 | |
| | Lump Sum Target Bonus Payment | | — | | | 285,000 | |
| | Health Insurance Premiums | | 26,571 | | | 39,856 | |
| | Vesting Acceleration(1) | | — | | | 2,403,330 | |
| | Benefit Total | | 501,571 | | | 3,440,686 | |
| Veer Bhavnagri | | Lump Sum Cash Severance Payment | | 464,000 | | | 696,000 | |
| | Lump Sum Target Bonus Payment | | — | | | 278,400 | |
| | Health Insurance Premiums | | 18,322 | | | 27,483 | |
| | Vesting Acceleration(1) | | — | | | 1,372,554 | |
| | Benefit Total | | 482,322 | | | 2,374,437 | |
(1) The value c/of the accelerated vesting of the outstanding stock options and restricted stock unit awards TPG Global, LLC, 301 Commerce Street, Suite 3300, Fort Worth, Texas 76102. This information is based on the closing market priceSchedule 13D/A filed on January 18, 2022 with the SEC.
(4) Based solely on information contained in a Schedule 13G as filed with the SEC on January 29, 2024 by BlackRock, Inc. (“BlackRock”). According to the Schedule 13G, BlackRock has sole voting power with respect to 9,787,819 shares and sole dispositive power with respect to 10,001,804 shares. The address of $14.92 per shareBlackRock is 50 Hudson Yards, New York, NY 10001. The Schedule 13G filed by BlackRock provides information only as of December 31, 2023. Because the information in the table above does not reflect any transactions between December 31, 2023 and March 31, 2024, BlackRock’s actual beneficial ownership of our common stock on DecemberMarch 31, 2021, less,2024 may be different than reported in the casetable above.
(5) Based solely on information contained in a Schedule 13G as filed with the SEC on February 13, 2024 by Vanguard Group (“Vanguard”). According to the Schedule 13G, Vanguard has shared voting power with respect to 56,409 shares, sole dispositive power with respect to 8,863,635 shares and shared dispositive power with respect to 167,190 shares. The address of Vanguard is 100 Vanguard Blvd., Malvern, PA 19355. The Schedule 13G filed by Vanguard provides information only as of December 29, 2023. Because the information in the table above does not reflect any transactions between December 29, 2023 and March 31, 2024, Vanguard’s actual beneficial ownership of our common stock on March 31, 2024 may be different than reported in the table above.
(6) Consists of the shares described in note (2) above.
(7) Consists of (i) 4,710,120 shares of common stock options, the exercise pricebeneficially owned by Rebecka Belldegrun, as beneficiary and trustee of the Bellco Legacy Trust fbo Rebecka Belldegrun, (ii) 195,039 shares of common stock beneficially owned by Bellco Legacy LLC, of which Dr. Belldegrun is a manager, (iii) 1,798,163 shares of common stock beneficially owned by Vida Ventures LLC (“Vida”), of which VV Manager LLC is the manager, of which Dr. Belldegrun is a Senior Managing Director, (iv) 432,518 shares of common stock held by Dr. Belldegrun, and (v) 2,847,489 shares of common stock issuable upon exercise of options, 1,414,559 of which will be unvested but exercisable within 60 days of March 31, 202 held by Dr. Belldegrun. The address of Dr. Belldegrun, Bellco Legacy Trust and Bellco Legacy LLC is 10100 Santa Monica Blvd., 15th Floor, Los Angeles, CA 90067. The address of Vida Ventures LLC is 40 Broad Street, #201, Boston, MA 02109.
(8) Consists of (i) 2,313,927 shares of common stock optionheld by David Chang, M.D., Ph.D., (ii) 1,201,108 shares subject to acceleration.of common stock held by the Chang 2006 Family Trust (“Chang Trust”), (iii) 856,044 shares of common stock held by the JEC 2019 Trust, (iv) 856,044 shares of common stock held by the RTC 2019 Trust, and (v) 4,820,438 shares of common stock issuable upon exercise of options, 2,125,511 of which will be unvested but exercisable within 60 days of March 31, 2024. Dr. Chang is co-trustee of the Chang Trust, JEC 2019 Trust and RTC 2019 Trust.
TRANSACTIONS WITH RELATED PERSONS(9) Consists of (i) 303,063 shares of common stock held by Joshua Kazam and (ii) 116,120 shares of common stock issuable upon exercise of options, 6,976 of which will be unvested but exercisable within 60 days of March 31, 2024.
(10) Consists of (i) 229,172 shares of common stock held by Franz Humer, Ph.D. and (ii) 183,750 shares of common stock issuable upon exercise of options.
(11) Consists of (i) 6,535 shares of common stock held by the Messemer Family Trust (“Messemer Trust”), (ii) 18,640 shares of common stock held by Ms. Messemer and (iii) 351,750 shares of common stock issuable upon exercise of options. Ms. Messemer is trustee of the Messemer Trust.
(12) Consists of (i) 255,551 shares of common stock held by Owen Witte, M.D. and (ii) 89,575 shares of common stock issuable upon exercise of options.
(13) Consists of 250,575 shares of common stock issuable upon exercise of options, 13,198 of which will be unvested but exercisable within 60 days of March 31, 2024.
(14) Consists of (i) 64,821 shares of common stock held by Vicki Sato, Ph.D., and (ii) 90,451 shares of common stock issuable upon exercise of options, 3,697 of which will be unvested but exercisable within 60 days of March 31, 2024.
(15) Consists of (i) 1,776 shares of common stock held by Stephen Mayo and (ii) 170,607 shares of common stock issuable upon exercise of options, 30,864 of which will be unvested but exercisable within 60 days of March 31, 2024.
(16) Consists of (i) 38,287 shares of common stock held by Zachary J Roberts, M.D. and (ii) 873,015 shares of common stock issuable upon exercise of options, 582,011 of which will be unvested but exercisable within 60 days of March 31, 2024.
(17) Consists of 960,854 shares of common stock issuable upon exercise of options, all of the options will be unvested but exercisable within 60 days of March 31, 2024.
(18) Consists of 831,024 shares of common stock issuable upon exercise of options, 605,956 of which will be unvested but exercisable within 60 days of March 31, 2024.
(19) Consists of (i) 190 shares of common stock held by Geoffrey Parker and (ii) 950,000 shares of common stock issuable upon exercise of options, all of the options will be unvested but exercisable within 60 days of March 31, 2024.
(20) Includes the shares described in notes (6) through (19) above.
| | | | | | | | |
Transactions with Related Persons
|
Related-Person Transactions policyPolicy and Procedures
We have adopted a written related-person transactions policy that sets forth our policies and procedures regarding the identification, review, consideration and oversight of “related-person transactions.” For purposes of our policy only, a “related-person transaction” is a transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which we and any “related person” are participants involving an amount that exceeds $120,000. Transactions involving compensation for services provided to us as an employee, consultant or director are not considered related-person transactions under this policy. A related person is any executive officer, director, nominee to become a director or a holder of more than 5% of our common stock, including any of their immediate family members and affiliates, including entities owned or controlled by such persons.
Under the policy, where a transaction has been identified as a related-person transaction, management must present information regarding the proposed related-person transaction to our Audit Committee (or, where review by our Audit Committee would be
inappropriate, to another independent body of our Board) for review. The presentation must include a description of, among other things, all of the parties thereto, the direct and indirect interests of the related persons, the purpose of the transaction, the material facts, the benefits of the transaction to us and whether any alternative transactions are available, an assessment of whether the terms are comparable to the terms available from unrelated third parties and management’s recommendation. To identify related-person transactions in advance, we rely on information supplied by our executive officers, directors and certain significant stockholders. In considering related-person transactions, our Audit Committee or another independent body of our Board takes into account the relevant available facts and circumstances including, but not limited to:
•the risks, costs and benefits to us;
•the impact on a director’s independence in the event the related person is a director, immediate family member of a director or an entity with which a director is affiliated;
•the terms of the transaction;
•the availability of other sources for comparable services or products; and
•the terms available to or from, as the case may be, unrelated third parties.
In the event a director has an interest in the proposed transaction, the director must recuse themselves from the deliberations and approval.
Certain Related-Person Transactions
The following sections summarize transactions since January 1, 20212022 to which we have been a party, in which the amount involved in the transaction exceeded the lesser of $120,000 or 1% of the average of the Company's total assets at year end for the last two completed fiscal years and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock, including any of their immediate family members and affiliates, including entities owned or controlled by such persons, had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive Compensation” and “Director Compensation.”
Pfizer Asset Purchase Transaction
PF Equity Holdings 2 B.V.
Pfizer held 22,032,040 shares of our common stock based on the Schedule 13D/A filed on September 17, 2021October 25, 2023 with the SEC. According to the Schedule 13D/A filing, PF Equity Holdings 2 B.V. is a wholly-owned subsidiary of Pfizer Inc. (“Pfizer”) formed for the purpose of holding certain assets owned or controlled by Pfizer or its direct or indirect subsidiaries. Based on a Form 4 filed on April 4, 2022 by PF Equity Holdings 2 B.V., Pfizer held the 22,032,040 shares as of March 31, 2022.
In April 2018, we entered into an Asset Contribution Agreement (the “Pfizer Agreement”) with Pfizer pursuant to which we acquired certain assets and assumed certain liabilities from Pfizer, including a Research Collaboration and License Agreement with Cellectis S.A. and an Exclusive License and Collaboration Agreement with Les Laboratoires Servier SAS and Institut de Recherches Internationales Servier SAS (collectively, “Servier”), and other intellectual property for the development and administration of CAR T cells for the treatment of cancer.
As consideration for the purchased assets, we issued Pfizer 3,187,772 shares of our Series A-1 Preferred Stock. In addition, we are required to make milestone payments upon successful completion of regulatory and sales milestones on a target-by-target basis for certain targets, including CD19 and BCMA, covered by the Pfizer Agreement. The aggregate potential milestone payments upon successful completion of various regulatory milestones in the United States and the European Union are $30 million or $60 million per target (depending on the target, and $840.0 million for all targets), provided that we are not obligated to pay a milestone for regulatory approval in the European Union for an anti-CD19 allogeneic CAR T cell product, to the extent Servier has commercial rights to such territory. The aggregate potential milestone payments upon reaching certain annual net sales thresholds in North America, Europe, Asia, Australia and Oceania, which we refer to as the Territory, for a certain number of targets covered by the Pfizer Agreement are $325.0 million per target. Concurrently with our entry into the Pfizer Agreement, we and Pfizer entered into a letter agreement pursuant to which Pfizer granted us, in partial consideration for our milestone and royalty payment obligations under the Pfizer Agreement, an option to expand the Territory to include some or all of the rest of the world at our election. We may exercise the option at any time during the 12 year period following closing of the asset acquisition under the Pfizer Agreement.
Pfizer is also eligible to receive, on a product-by-product and country-by-country basis, (i) royalties in the low single-digit percentage on annual net sales in the United States for products commercialized by us targeting certain targets, including CD19, covered by the Pfizer Agreement, and (ii) tiered marginal royalties ranging from the low to mid-single-digit percentages on annual net sales in any country in the world for products commercialized by us targeting certain other targets covered by the Pfizer Agreement and (iii) royalties in the low single-digit percentage on annual net sales in any country in the Territory for products commercialized by us
targeting targets not covered by the Pfizer Agreement that use certain Pfizer intellectual property and for which an IND is first filed on or before April 6, 2023.Agreement. The royalties in the foregoing clauses (i) and (ii) are subject to reduction for products not covered by certain patent claims or for future required licenses of third party intellectual property. Our royalty obligation with respect to a given product in a given country, which we refer to as the Pfizer Royalty Term, begins upon the first sale of such product in such country and ends on the later of (i) expiration of the last claim of a defined set of patent rights, in each case covering such product in such country or (ii) 12 years from the first sale of such product in such country.
Under the Pfizer Agreement, we are required to use commercially reasonable efforts to develop and seek regulatory approval in and for the United States and the European Union for certain products covered by the Pfizer Agreement and to commercialize each product covered by the Pfizer Agreement in the applicable royalty territory in which regulatory approval for such product has been obtained. We also agreed to offer employment to certain Pfizer employees on terms no less favorable than the terms such employees enjoyed while being employed by Pfizer. We hired 39 employees from Pfizer pursuant to the terms of the Pfizer Agreement.
Pfizer is required, subject to certain limitations, to indemnify us against damages arising out of any breach or inaccuracy in the representations or warranties made by Pfizer, any breach of a covenant by Pfizer or any liability not acquired by us. Likewise, we are required, subject to certain limitations, to indemnify Pfizer against damages arising out of any breach or inaccuracy of our representations and warranties, any breach of a covenant made in the agreement or the related patent and know-how license agreement by us, including any practice of intellectual property outside of the scope of the license granted to us, or any assumed liability.
During the year ended December 31, 2021, the Company sold $0.1 million in excess raw materials to Pfizer.
Investors’ Rights Agreement
Pursuant to an investors’ rights agreement, dated April 6, 2018, as amended, between us and certain of our stockholders, including certain holders of more than five percent of our capital stock and entities affiliated with certain of our directors, Dr. Chang, Mr. Kazam, Dr. Belldegrun, Dr. Witte and Dr. Humer, each directors of our company, as well as Pfizer, entities affiliated with TPG Carthage Holdings, L.P. and entities affiliated with VVAG Special Fund LLC, are entitled to certain demand registration rights, piggyback registration rights and Form S-3 registration rights.
Consulting Arrangements
Two River, LLC
In June 2018, we entered into a consulting agreement with Two River, LLC (“Two River”). Arie Belldegrun, M.D., the Executive Chair of our Board and Joshua Kazam, a member of our Board, are each partners of Two River, and David Chang, M.D., Ph.D., our President and Chief Executive Officer, is a venture partner of Two River. Pursuant to the consulting agreement, Two River providesprovided strategic, financial, business development and secretarial consulting services and iswas compensated for such services. We paid Two River an aggregate of $0.6$0.7 million and $0.3 million in consulting fees in 2021.2022 and 2023, respectively. Dr. Belldegrun and Dr. Chang do not receive any salary, commission or other fees for serving as partners of Two River. We have terminated the Two River consulting agreement effective December 31, 2023.
Bellco Capital LLC
In August 2018, we entered into a consulting agreement with Bellco Capital LLC (“Bellco”), which was amended in January 2019, January 2020, January 2021 and January 2022 (“Bellco Consulting Agreement”). Bellco is owned by our Executive Chair, Arie Belldegrun, M.D., as co-trustee of the Belldegrun Family Trust, and Dr. Rebecka Belldegrun, as co-trustee of the Belldegrun Family Trust, and Dr. Arie Belldegrun is the Manager of Bellco. Pursuant to the consulting agreement, Bellco provides certain consulting services for us, which are performed by Dr. Belldegrun and include without limitation, providing advice and analysis with respect to our business, business strategy and potential opportunities in the field of allogeneic CAR T cell therapy and any other aspect of the CAR T cell therapy business as we may agree. Arie Belldegrun. In
consideration for these services, we paid Bellco $38,583$40,217 per month in 2021.2022. In recognition of the extraordinary performance of Bellco and in helping us meet our 20212022 corporate goals, an annual performance award in an amount of $0.3$0.2 million was paid to Bellco in 2022.2023. The Company also increasedmade no changes to the monthly payment to Bellco for 2022 to $40,217. We2023. We also reimburse Bellco for out of pocket expenses incurred in performing the services.
Employment Arrangements
We currently have written employment letter agreements with our executive officers. For information about our employment agreements with our named executive officers, refer The compensation paid to Bellco in 2023 under the consulting agreement is described above under the heading “Executive Compensation—Agreements with our Named Executive Officers.”Chair Compensation” and is incorporated herein by reference.
Sublease Agreement
In December 2018, we subleased approximately 1,290entered into a sublease with Bellco for 1,293 square feet of office space in Los Angeles, California for a three year term. On April 1, 2020, Bellco Capital Advisors Inc. assumed all rights, title, interests and obligations under the sublease from Bellco. TheBellco Capital LLC. In November 2021, the sublease has a three-year term, which was extended to June 30, 2025,2025. The sublease was amended, effective in July 2022, to move to a nearby location, with office space of 737 square feet. Our executive chair, Arie Belldegrun, M.D., is a trustee of the Belldegrun Family Trust, which controls Bellco Capital Advisors Inc. In each of 2022 and 2023, we made $0.1 million of rent payments in accordance with this sublease. In 2023, we exercised our early termination right under the sublease agreement and the sublease was terminated effective December 31, 2023.
In February 2023, we entered into a new sublease with Bellco for 2,218 square feet of office space in Los Angeles, California. The sublease term is 115 months, subject to certain early termination rights. Commencing in September 2018, we payThe sublease commenced on January 1, 2024. We paid approximately $0.2 million towards the monthly base rent due for the first month of approximately $6,500, increasing 3.5% per year,the sublease term and our share of the security deposit. The total estimated amount of base rent is $3.4 million, subject to rent abatement from November 2018 through May 2019. The monthly base rent will total approximately $220,000 for the full term of the sublease.abatement. We
also contributedexpect to contribute to certain tenant improvements to the space totaling approximately $75,000.to its share of the total tenant contribution. The monthly base rent and related occupancy costs are pass-through costs to the master landlord or other third parties and no portion is retained by Bellco.
Stock Options Granted to Executive Officers and Directors
We have granted stock options to our executive officers and directors, as more fully described in “Executive Compensation – 2021 Outstanding Equity Awards Table.”
Indemnification Agreements
We have entered into, and intend to continue to enter into, separate indemnification agreements with our directors and executive officers, in addition to the indemnification provided for in our amendedAmended and restated bylaws.Restated Bylaws. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or executive officers or any other company or enterprise to which the person provides services at our request. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability and indemnification provisions in our amendedAmended and restated certificateRestated Certificate of incorporationIncorporation and amendedAmended and restated bylawsRestated Bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Director Independence
For information regarding our director and committee member independence, please see
| | | | | | | | |
Proposal 3
Ratification of Selection of Independent Registered Public Accounting Firm
|
Recommendation:
The Board recommends the sections above headed “Information Regardingvote “FOR” the ratification of the selection by the Audit Committee of the Board of Directors and Corporate Governance—Independenceof Ernst & Young LLP as our independent registered public accounting firm for our fiscal year ending December 31, 2024.
The Audit Committee of the Board of Directors”Directors has appointed Ernst & Young LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2024 and “Information Regardinghas further directed that management submit the selection of its independent registered public accounting firm for ratification by the stockholders at the Annual Meeting. Ernst & Young LLP has audited the Company’s financial statements since its inception in 2017. Representatives of Ernst & Young LLP are expected to be present at the Annual Meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither the Company’s Amended and Restated Bylaws nor other governing documents or law require stockholder ratification of the selection of Ernst & Young LLP as the Company’s independent registered public accounting firm. However, the Audit Committee of the Board is submitting the selection of Ernst & Young LLP to the stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, the Audit Committee of the Board will reconsider whether or not to retain that firm. Even if the selection is ratified, the Audit Committee of the Board in its discretion may direct the appointment of a different independent registered public accounting firm at any time during the year if they determine that such a change would be in the best interests of the Company and its stockholders.
The affirmative vote of the holders of a majority of the shares present at the Annual Meeting or represented by proxy and entitled to vote on the matter at the Annual Meeting will be required to ratify the selection of the accounting firm. Abstentions will have the same effect as an “against” vote on this proposal.
Principal Accountant Fees and Services
The following table represents aggregate fees billed to the Company for the fiscal years ended December 31, 2023 and December 31, 2022 by Ernst & Young LLP, the Company’s principal accountant.
| | | | | | | | | | | |
| Fiscal Year Ended |
| 2023 | | 2022 |
| (in thousands) |
| Fee Category | | | |
| Audit fees(1) | $ | 1,680 | | $ | 1,577 |
| Audit-related fees | — | | — |
| Tax fees | — | | — |
| All other fees(2) | 2 | | 1 |
| Total fees | $ | 1,682 | | $ | 1,578 |
(1) Audit fees consist of fees for professional services provided primarily in connection with the annual audit of our financial statements, audit of our internal controls over financial reporting, quarterly reviews and services associated with SEC registration statements and other documents issued in connection with the Company’s at the market offerings, including comfort letters and consents.
(2) All other fees consist of a subscription to Ernst & Young Atlas Online, a proprietary knowledge management and research system.
All fees described above were pre-approved by the Audit Committee.
Pre-Approval Policies and Procedures
Pursuant to its charter, the Audit Committee must review and approve, in advance, the scope and plans for the audits and the audit fees and approve in advance (or, where permitted under the rules and regulations of the SEC, subsequently) all non-audit services to be performed by the independent registered public accounting firm that are not otherwise prohibited by law and any associated fees. The Audit Committee may delegate to one or more members of the committee the authority to pre-approve audit and permissible non-audit services, as long as this pre-approval is presented to the full committee at scheduled meetings.
Report of the Audit Committee of the Board of Directors *
The Audit Committee has reviewed and Corporate Governance—Information Regarding Committeesdiscussed the audited financial statements for the fiscal year ended December 31, 2023 with management of the Company. The Audit Committee has discussed with the independent registered public accounting firm the matters required to be discussed by the applicable requirements of the Public Company Accounting Oversight Board (“PCAOB”) and the SEC. The Audit Committee has also received the written disclosures and the letter from the independent registered public accounting firm required by applicable requirements of the PCAOB regarding the independent accountants’ communications with the Audit Committee concerning independence, and has discussed with the independent registered public accounting firm the accounting firm’s independence. Based on the foregoing, the Audit Committee recommended to the Board of Directors that the audited financial statements be included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023.
Members of Allogene Therapeutics, Inc.
Audit Committee
Ms. Deborah Messemer (Chair)
Ms. Elizabeth Barrett
Dr. Franz Humer, Ph.D.
* The material in this report is not “soliciting material,” which areis not deemed “filed” with the SEC and is not to be incorporated into this section headed “Transactions with Related Persons” by reference.reference in any filing of the Company under the Exchange Act or the Securities Act of 1933, as amended (the “Securities Act”), whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
| | | | | | | | |
Householding of Proxy Materials
|
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for Notices of Internet Availability of Proxy Materials or other annual meeting materials with respect to two or more stockholders sharing the same address by delivering a single Notice of Internet Availability of Proxy Materials or other annual meeting materials addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
This year, a number of brokers with account holders who are Company stockholders will be “householding” the Company’s proxy materials. A single Notice of Internet Availability of Proxy Materials will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate Notice of Internet Availability of Proxy Materials, please notify your broker or the Company. Direct your written request to Allogene Therapeutics, Inc., Corporate Secretary, 210 East Grand Avenue, South San Francisco, California 94080, or call us at (650) 457-2700. Stockholders who currently receive multiple copies of the Notices of Internet Availability of Proxy Materials at their addresses and would like to request “householding” of their communications should contact their brokers.
The Board of Directors knows of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the meeting, it is the intention of the persons named in the accompanying proxy to vote on such matters in accordance with their best judgment.
By Order of the Board of Directors
David M. Tanen
Earl Douglas
Senior Vice President, General Counsel & Corporate Secretary
South San Francisco, California
April , 202223, 2024
A copy of the Company’s Annual Report to the Securities and Exchange Commission on Form 10-K for the fiscal year ended December 31, 20212023 is available without charge upon written request to: Corporate Secretary, Allogene Therapeutics, Inc., 210 East Grand Avenue, South San Francisco, California 94080.
APPENDIX A
CERTIFICATE OF AMENDMENT OF THE
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION OF
ALLOGENE THERAPEUTICS, INC.
ALLOGENE THERAPEUTICS, INC., a corporation organized and existing under and by virtue of the General Corporation Law of the State of Delaware (the “Company”), does hereby certify:
FIRST: The name of the Company is Allogene Therapeutics, Inc. and the date of filing of the Company’s original Certificate of Incorporation with the Secretary of State of the State of Delaware was November 30, 2017.
SECOND: The Board of Directors of the Company, acting in accordance with the provisions of Sections 141 and 242 of the General Corporation Law of the State of Delaware (the “DGCL”), adopted resolutions amending its Amended and Restated Certificate of Incorporation (the “Restated Certificate”), as follows:
Paragraph A of Article IV of the Restated Certificate is hereby amended and restated to read in its entirety as follows:
“A. The Company is authorized to issue two classes of stock to be designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares which the Company is authorized to issue is 410,000,000 shares. 400,000,000 shares shall be Common Stock, each having a par value of $0.001. 10,000,000 shares shall be Preferred Stock, each having a par value of $0.001.”
THIRD: This Certificate of Amendment has been duly adopted and approved by the stockholders of the Company in accordance with Sections 211 and 242 of the DGCL.
FOURTH: This Certificate of Amendment shall become effective upon filing with the Secretary of State of the State of Delaware.
IN WITNESS WHEREOF, Allogene Therapeutics, Inc. has caused this Certificate of Amendment to be signed by its President and Chief Executive Officer on , 2022.
| | | | | | | | | | | |
| ALLOGENE THERAPEUTICS, INC. | |
| | |
| By: | | |
| | David Chang, M.D., Ph.D. | |
| | President, Chief Executive Officer | |